Organotypic slice culture of the hypothalamic paraventricular nucleus of rat
- PMID: 17322769
- PMCID: PMC2872692
- DOI: 10.4142/jvs.2007.8.1.15
Organotypic slice culture of the hypothalamic paraventricular nucleus of rat
Abstract
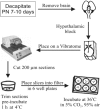
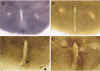
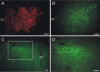
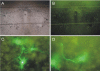
Organotypic slice cultures have been developed as an alternative to acute brain slices because the neuronal viability and synaptic connectivity in these cultures can be preserved well for a prolonged period of time. This study evaluated a stationary organotypic slice culture developed for the hypothalamic paraventricular nucleus (PVN) of rat. The results showed that the slice cultures maintain the typical shape of the nucleus, the immunocytochemical signals for oxytocin, vasopressin, and corticotropin-releasing hormone, and the electrophysiological properties of PVN neurons for up to 3 weeks in vitro. The PVN neurons in the culture expressed the green fluorescent protein gene that had been delivered by the adenoviral vectors. The results indicate that the cultured slices preserve the properties of the PVN neurons, and can be used in longterm studies on these neurons in vitro.
Figures

Similar articles
-
Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding.Endocrinology. 2008 Mar;149(3):1295-301. doi: 10.1210/en.2007-1276. Epub 2007 Nov 29. Endocrinology. 2008. PMID: 18048495
-
Inhibition of oxytocin and vasopressin neuron activity in rat hypothalamic paraventricular nucleus by relaxin-3-RXFP3 signalling.J Physiol. 2017 Jun 1;595(11):3425-3447. doi: 10.1113/JP273787. Epub 2017 Feb 27. J Physiol. 2017. PMID: 28098344 Free PMC article.
-
Colocalization of dopamine receptors in BDNF-expressing peptidergic neurons in the paraventricular nucleus of rats.J Chem Neuroanat. 2020 Jul;106:101794. doi: 10.1016/j.jchemneu.2020.101794. Epub 2020 Apr 19. J Chem Neuroanat. 2020. PMID: 32315740
-
Synaptic Inputs of Neural Afferent Pathways to Vasopressin- and Oxytocin-Secreting Neurons of Supraoptic and Paraventricular Hypothalamic Nuclei.Endocr Metab Immune Disord Drug Targets. 2016;16(4):276-287. doi: 10.2174/1871530317666170104124229. Endocr Metab Immune Disord Drug Targets. 2016. PMID: 28056741 Review.
-
Dynamism of chemoarchitecture in the hypothalamic paraventricular nucleus.Brain Res Bull. 1988 Jun;20(6):699-708. doi: 10.1016/0361-9230(88)90080-9. Brain Res Bull. 1988. PMID: 3044519 Review.
Cited by
-
Effects of blast overpressure on neurons and glial cells in rat organotypic hippocampal slice cultures.Front Neurol. 2015 Feb 12;6:20. doi: 10.3389/fneur.2015.00020. eCollection 2015. Front Neurol. 2015. PMID: 25729377 Free PMC article.
-
Photoswitchable Probes of Oxytocin and Vasopressin.J Med Chem. 2023 Nov 9;66(21):14853-14865. doi: 10.1021/acs.jmedchem.3c01415. Epub 2023 Oct 19. J Med Chem. 2023. PMID: 37857356 Free PMC article.
-
Organotypic Spinal Cord Culture: a Proper Platform for the Functional Screening.Mol Neurobiol. 2016 Sep;53(7):4659-74. doi: 10.1007/s12035-015-9403-z. Epub 2015 Aug 27. Mol Neurobiol. 2016. PMID: 26310972 Review.
References
-
- Arima H, House SB, Gainer H, Aguilera G. Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro. Endocrinology. 2002;143:4165–4171. - PubMed
-
- Armstrong WE. Hypothalamic supraoptic and paraventricular nuclei. In: Paxinos G, editor. The Rat Nervous System. 3rd. San Diego: Elsevier Academic Press; 2004. pp. 369–388.
-
- Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ. Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci. 2004;19:777–782. - PubMed
-
- Belenky M, Wagner S, Yarom Y, Matzner H, Cohen S, Castel M. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience. 1996;70:127–143. - PubMed
-
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

