Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen
- PMID: 17322771
- PMCID: PMC2872694
- DOI: 10.4142/jvs.2007.8.1.27
Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen
Abstract
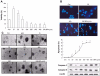
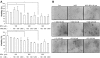
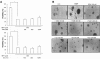
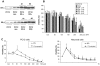
Bisphenol A (BPA), a ubiquitous environmental contaminant, has been shown to cause developmental toxicity and carcinogenic effects. BPA may have physiological activity through estrogen receptor (ER) -alpha and -beta, which are expressed in the central nervous system. We previously found that exposure of BPA to immature mice resulted in behavioral alternation, suggesting that overexposure of BPA could be neurotoxic. In this study, we further investigated the molecular neurotoxic mechanisms of BPA. BPA increased vulnerability (decrease of cell viability and differentiation, and increase of apoptotic cell death) of undifferentiated PC12 cells and cortical neuronal cells isolated from gestation 18 day rat embryos in a concentration-dependent manner (more than 50 microM). The ER antagonists, ICI 182,780, and tamoxifen, did not block these effects. The cell vulnerability against BPA was not significantly different in the PC12 cells overexpressing ER-alpha and ER-beta compared with PC12 cells expressing vector alone. In addition, there was no difference observed between BPA and 17-beta estradiol, a well-known agonist of ER receptor in the induction of neurotoxic responses. Further study of the mechanism showed that BPA significantly activated extracellular signal-regulated kinase (ERK) but inhibited anti-apoptotic nuclear factor kappa B (NF-kappaB) activation. In addition, ERK-specific inhibitor, PD 98,059, reversed BPA-induced cell death and restored NF-kappaB activity. This study demonstrated that exposure to BPA can cause neuronal cell death which may eventually be related with behavioral alternation in vivo. However, this neurotoxic effect may not be directly mediated through an ER receptor, as an ERK/NF-kappaB pathway may be more closely involved in BPA-induced neuronal toxicity.
Figures

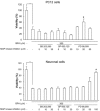

Similar articles
-
Effects of the environmental estrogens bisphenol A, o,p'-DDT, p-tert-octylphenol and coumestrol on apoptosis induction, cell proliferation and the expression of estrogen sensitive molecular parameters in the human breast cancer cell line MCF-7.J Steroid Biochem Mol Biol. 2002 Jan;80(1):61-70. doi: 10.1016/s0960-0760(01)00173-x. J Steroid Biochem Mol Biol. 2002. PMID: 11867264
-
Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity.Cell Death Dis. 2013 Jun 20;4(6):e676. doi: 10.1038/cddis.2013.203. Cell Death Dis. 2013. PMID: 23788033 Free PMC article.
-
MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells.Inflammation. 2015 Apr;38(2):637-48. doi: 10.1007/s10753-014-9971-5. Inflammation. 2015. PMID: 25047101
-
Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure.Endocrinology. 2006 Jun;147(6 Suppl):S56-69. doi: 10.1210/en.2005-1159. Epub 2006 May 11. Endocrinology. 2006. PMID: 16690810 Review.
-
Nongenomic signaling pathways of estrogen toxicity.Toxicol Sci. 2010 May;115(1):1-11. doi: 10.1093/toxsci/kfp288. Epub 2009 Dec 2. Toxicol Sci. 2010. PMID: 19955490 Free PMC article. Review.
Cited by
-
Relationships between urinary biomarkers of phytoestrogens, phthalates, phenols, and pubertal stages in girls.Adolesc Health Med Ther. 2012 Jan 6;3:17-26. doi: 10.2147/AHMT.S15947. eCollection 2012. Adolesc Health Med Ther. 2012. PMID: 24600283 Free PMC article. Review.
-
Microarray expression profiling and co-expression network analysis of circulating LncRNAs and mRNAs associated with neurotoxicity induced by BPA.Environ Sci Pollut Res Int. 2018 May;25(15):15006-15018. doi: 10.1007/s11356-018-1678-y. Epub 2018 Mar 18. Environ Sci Pollut Res Int. 2018. PMID: 29552716
-
Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs.Neurotox Res. 2013 Apr;23(3):249-59. doi: 10.1007/s12640-012-9353-4. Epub 2012 Sep 21. Neurotox Res. 2013. PMID: 22996013
-
GGA1 participates in spermatogenesis in mice under stress.PeerJ. 2023 Aug 3;11:e15673. doi: 10.7717/peerj.15673. eCollection 2023. PeerJ. 2023. PMID: 37551344 Free PMC article.
-
Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway.Toxicol Sci. 2011 Jan;119(1):209-17. doi: 10.1093/toxsci/kfq319. Epub 2010 Oct 18. Toxicol Sci. 2011. PMID: 20956811 Free PMC article.
References
-
- Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002;937:1–7. - PubMed
-
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor and estrogen receptor to partial estrogen agonist/ antagonists. Mol Pharmacol. 1998;54:105–112. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

