Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients
- PMID: 17324241
- PMCID: PMC2000639
- DOI: 10.1111/j.1365-2125.2007.02862.x
Population pharmacokinetics of melphalan in paediatric blood or marrow transplant recipients
Abstract
Aim: To develop a population pharmacokinetic model for melphalan in children with malignant diseases and to evaluate limited sampling strategies for melphalan.
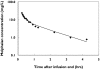
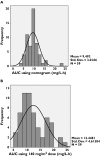
Methods: Melphalan concentration data following a single intravenous dose were collected from 59 children with malignant diseases aged between 0.3 and 18 years. The data were split into two sets: the model development dataset (39 children, 571 concentration observations) and the model validation dataset (20 children, 277 concentration observations). Population pharmacokinetic modelling was performed with the NONMEM software. Stepwise multiple linear regression was used to develop a limited sampling model for melphalan.
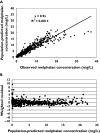
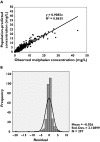
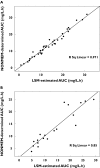
Results: A two-compartment model was fitted to the concentration-vs.-time data. The following covariate population pharmacokinetic models were obtained: (i) Clearance (l h(-1)) = 0.34.WT - 3.17.CPT + 0.0377.GFR, where WT = weight (kg), CPT = prior carboplatin therapy (0 = no, 1 = yes), and GFR = glomerular filtration rate (ml min(-1) 1.73 m(-2)); (ii) Volume of distribution (l) = 1.12 + 0.178.WT. Interpatient variability (coefficient of variation) was 27.3% for clearance and 33.8% for volume of distribution. There was insignificant bias and imprecision between observed and model-predicted melphalan concentrations in the validation dataset. A three-sample limited sampling model was developed which adequately predicted the area under the concentration-time curve (AUC) in the development and validation datasets.
Conclusions: A population pharmacokinetic model for melphalan has been developed and validated and may now be used in conjunction with pharmacodynamic data to develop safe and effective dosing guidelines in children with malignant diseases.
Figures
References
-
- Shaw PJ, Pinkerton CR, Yaniv I. Melphalan combined with carboplatin dose based on glomerular filtration rate followed by autologous stem cell rescue for children with solid tumours. Bone Marrow Transplant. 1996;16:401–5. - PubMed
-
- Michel G, Maraninchi D, Demeocq F, Perrimond H, Blaise D, Gaspard MH, Stoppa AM, Gastaut JA, Lepeu G, Novakovitch G, Marguerite G, Carcassonne Y. Repeated courses of high dose melphalan and unpurged autologous bone marrow transplantation in children with acute non-lymphoblastic leukemia in first complete remission. Bone Marrow Transplant. 1998;3:105–11. - PubMed
-
- Cornwell GG, III, Pajak TF, McIntyre OR, Kochwa S, Dosik H. Influence of renal failure on myelosuppressive effects of melphalan: cancer and leukemia group B experience. Cancer Treat Rep. 1982;66:475–81. - PubMed
-
- Moreau P, Kergueris M-F, Milpied N, Le Tortorec SL, Mahe B, Bulabois C-E, Rapp M-J, Larousse C, Bataille R, Harousseau J-L. A pilot study of 220 mg/m2 melphalan followed by autologous stem cell transplantation in patients with advanced haematological malignancies: pharmacokinetics and toxicity. Br J Haematol. 1996;95:527–30. - PubMed
-
- Sarosy G, Leyland-Jones B, Soochan P, Cheson BD. The systemic administration of intravenous melphalan. J Clin Oncol. 1988;6:1768–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

