Elevating the levels of Sox2 in embryonal carcinoma cells and embryonic stem cells inhibits the expression of Sox2:Oct-3/4 target genes
- PMID: 17324942
- PMCID: PMC1874607
- DOI: 10.1093/nar/gkm059
Elevating the levels of Sox2 in embryonal carcinoma cells and embryonic stem cells inhibits the expression of Sox2:Oct-3/4 target genes
Abstract
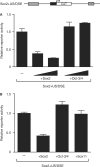
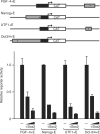
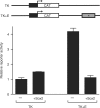
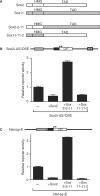
Recent studies have identified large sets of genes in embryonic stem and embryonal carcinoma cells that are associated with the transcription factors Sox2 and Oct-3/4. Other studies have shown that Sox2 and Oct-3/4 work together cooperatively to stimulate the transcription of their own genes as well as a network of genes required for embryogenesis. Moreover, small changes in the levels of Sox2:Oct-3/4 target genes alter the fate of stem cells. Although positive feedforward and feedback loops have been proposed to explain the activation of these genes, little is known about the mechanisms that prevent their overexpression. Here, we demonstrate that elevating Sox2 levels inhibits the endogenous expression of five Sox2:Oct-3/4 target genes. In addition, we show that Sox2 repression is dependent on the binding sites for Sox2 and Oct-3/4. We also demonstrate that inhibition is dependent on the C-terminus of Sox2, which contains its transactivation domain. Finally, our studies argue that overexpression of neither Oct-3/4 nor Nanog broadly inhibits Sox2:Oct-3/4 target genes. Collectively, these studies provide new insights into the diversity of mechanisms that control Sox2:Oct-3/4 target genes and argue that Sox2 functions as a molecular rheostat for the control of a key transcriptional regulatory network.
Figures





Similar articles
-
Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells.Nat Cell Biol. 2007 Jun;9(6):625-35. doi: 10.1038/ncb1589. Epub 2007 May 21. Nat Cell Biol. 2007. PMID: 17515932
-
Identification of DPPA4 and other genes as putative Sox2:Oct-3/4 target genes using a combination of in silico analysis and transcription-based assays.J Cell Physiol. 2008 Sep;216(3):651-62. doi: 10.1002/jcp.21440. J Cell Physiol. 2008. PMID: 18366076
-
Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells.Stem Cells. 2008 Apr;26(4):903-11. doi: 10.1634/stemcells.2007-0951. Epub 2008 Jan 31. Stem Cells. 2008. PMID: 18238855
-
Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation.Int J Dev Biol. 2004;48(8-9):819-27. doi: 10.1387/ijdb.041868hk. Int J Dev Biol. 2004. PMID: 15558474 Review.
-
Sox2 and Oct-3/4: a versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells.Wiley Interdiscip Rev Syst Biol Med. 2009 Sep-Oct;1(2):228-236. doi: 10.1002/wsbm.12. Wiley Interdiscip Rev Syst Biol Med. 2009. PMID: 20016762 Free PMC article. Review.
Cited by
-
SRY (sex determining region Y)-box2 (Sox2)/poly ADP-ribose polymerase 1 (Parp1) complexes regulate pluripotency.Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3772-7. doi: 10.1073/pnas.1108595109. Epub 2012 Feb 23. Proc Natl Acad Sci U S A. 2012. PMID: 22362888 Free PMC article.
-
Banf1 is required to maintain the self-renewal of both mouse and human embryonic stem cells.J Cell Sci. 2011 Aug 1;124(Pt 15):2654-65. doi: 10.1242/jcs.083238. Epub 2011 Jul 12. J Cell Sci. 2011. PMID: 21750191 Free PMC article.
-
Transcription factors and neural stem cell self-renewal, growth and differentiation.Cell Adh Migr. 2009 Oct-Dec;3(4):412-24. doi: 10.4161/cam.3.4.8803. Epub 2009 Oct 27. Cell Adh Migr. 2009. PMID: 19535895 Free PMC article. Review.
-
sox2 and sox3 Play unique roles in development of hair cells and neurons in the zebrafish inner ear.Dev Biol. 2018 Mar 1;435(1):73-83. doi: 10.1016/j.ydbio.2018.01.010. Epub 2018 Jan 31. Dev Biol. 2018. PMID: 29355523 Free PMC article.
-
Distinct molecular profile and restricted stem cell potential defines the prospective human cranial neural crest from embryonic stem cell state.Stem Cell Res. 2020 Dec;49:102086. doi: 10.1016/j.scr.2020.102086. Epub 2020 Nov 11. Stem Cell Res. 2020. PMID: 33370869 Free PMC article.
References
-
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006;38:431–440. - PubMed
-
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. - PubMed
-
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

