CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation
- PMID: 17325034
- PMCID: PMC1900023
- DOI: 10.1128/MCB.01352-06
CSK controls retinoic acid receptor (RAR) signaling: a RAR-c-SRC signaling axis is required for neuritogenic differentiation
Abstract
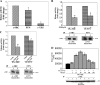
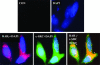
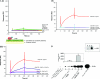
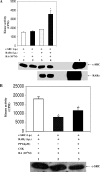
Herein, we report the first evidence that c-SRC is required for retinoic acid (RA) receptor (RAR) signaling, an observation that suggests a new paradigm for this family of nuclear hormone receptors. We observed that CSK negatively regulates RAR functions required for neuritogenic differentiation. CSK overexpression inhibited RA-mediated neurite outgrowth, a result which correlated with the inhibition of the SFK c-SRC. Consistent with an extranuclear effect of CSK on RAR signaling and neurite outgrowth, CSK overexpression blocked the downstream activation of RAC1. The conversion of GDP-RAC1 to GTP-RAC1 parallels the activation of c-SRC as early as 15 min following all-trans-retinoic acid treatment in LA-N-5 cells. The cytoplasmic colocalization of c-SRC and RARgamma was confirmed by immunofluorescence staining and confocal microscopy. A direct and ligand-dependent binding of RAR with SRC was observed by surface plasmon resonance, and coimmunoprecipitation studies confirmed the in vivo binding of RARgamma to c-SRC. Deletion of a proline-rich domain within RARgamma abrogated this interaction in vivo. CSK blocked the RAR-RA-dependent activation of SRC and neurite outgrowth in LA-N-5 cells. The results suggest that transcriptional signaling events mediated by RA-RAR are necessary but not sufficient to mediate complex differentiation in neuronal cells. We have elucidated a nongenomic extranuclear signal mediated by the RAR-SRC interaction that is negatively regulated by CSK and is required for RA-induced neuronal differentiation.
Figures





Similar articles
-
Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor.Mol Endocrinol. 2007 Oct;21(10):2391-402. doi: 10.1210/me.2007-0062. Epub 2007 Jun 26. Mol Endocrinol. 2007. PMID: 17595318
-
Functional interference between retinoic acid or steroid hormone receptors and the oncoprotein Fli-1.Oncogene. 1997 Dec 18;15(25):3067-82. doi: 10.1038/sj.onc.1201503. Oncogene. 1997. PMID: 9444955
-
Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation.Oncogene. 2006 Sep 7;25(40):5495-506. doi: 10.1038/sj.onc.1209554. Epub 2006 Apr 24. Oncogene. 2006. PMID: 16636672
-
Retinoic acid signaling and mouse embryonic stem cell differentiation: Cross talk between genomic and non-genomic effects of RA.Biochim Biophys Acta. 2015 Jan;1851(1):66-75. doi: 10.1016/j.bbalip.2014.04.003. Epub 2014 Apr 24. Biochim Biophys Acta. 2015. PMID: 24768681 Review.
-
Protein tyrosine kinases Src and Csk: a tail's tale.Curr Opin Chem Biol. 2003 Oct;7(5):580-5. doi: 10.1016/j.cbpa.2003.08.009. Curr Opin Chem Biol. 2003. PMID: 14580561 Review.
Cited by
-
The PI-3 kinase-Akt-MDM2-survivin signaling axis in high-risk neuroblastoma: a target for PI-3 kinase inhibitor intervention.Cancer Chemother Pharmacol. 2011 Aug;68(2):325-35. doi: 10.1007/s00280-010-1486-7. Epub 2010 Oct 24. Cancer Chemother Pharmacol. 2011. PMID: 20972874 Free PMC article.
-
RAC1 Takes the Lead in Solid Tumors.Cells. 2019 Apr 26;8(5):382. doi: 10.3390/cells8050382. Cells. 2019. PMID: 31027363 Free PMC article. Review.
-
Targeting to the non-genomic activity of retinoic acid receptor-gamma by acacetin in hepatocellular carcinoma.Sci Rep. 2017 Mar 23;7(1):348. doi: 10.1038/s41598-017-00233-5. Sci Rep. 2017. PMID: 28336971 Free PMC article.
-
Inhibition of the redox function of APE1/Ref-1 in myeloid leukemia cell lines results in a hypersensitive response to retinoic acid-induced differentiation and apoptosis.Exp Hematol. 2010 Dec;38(12):1178-88. doi: 10.1016/j.exphem.2010.08.011. Epub 2010 Sep 6. Exp Hematol. 2010. PMID: 20826193 Free PMC article.
-
The Fox and the Rabbits-Environmental Variables and Population Genetics (1) Replication Problems in Association Studies and the Untapped Power of GWAS (2) Vitamin A Deficiency, Herpes Simplex Reactivation and Other Causes of Alzheimer's Disease.ISRN Neurol. 2011;2011:394678. doi: 10.5402/2011/394678. Epub 2011 Jul 12. ISRN Neurol. 2011. PMID: 22389816 Free PMC article.
References
-
- Alsayed, Y., S. Uddin, N. Mahmud, F. Lekmine, D. V. Kalvakolanu, S. Minucci, G. Bokoch, and L. C. Platanias. 2001. Activation of Rac1 and the p38 mitogen-activated protein kinase pathway in response to all-trans-retinoic acid. J. Biol. Chem. 276:4012-4019. - PubMed
-
- Altucci, L., and H. Gronemeyer. 2001. The promise of retinoids to fight against cancer. Nat. Rev. Cancer 1:181-193. - PubMed
-
- Andrews, P. W. 1984. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 103:285-293. - PubMed
-
- Aoki, K., T. Nakamura, and M. Matsuda. 2004. Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells. J. Biol. Chem. 279:713-719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
