Ligand binding to the androgen receptor induces conformational changes that regulate phosphatase interactions
- PMID: 17325038
- PMCID: PMC1899975
- DOI: 10.1128/MCB.02411-06
Ligand binding to the androgen receptor induces conformational changes that regulate phosphatase interactions
Abstract
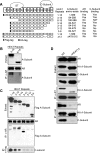
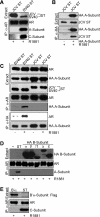
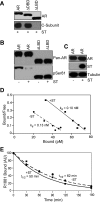
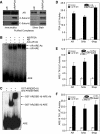
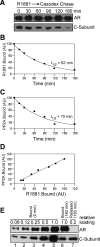
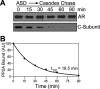
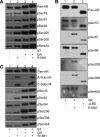
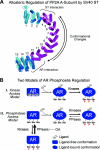
We describe a mechanism for protein phosphatase 2A (PP2A) targeting to the androgen receptor (AR) and provide insight into the more general issue of kinase and phosphatase interactions with AR. Simian virus 40 (SV40) small t antigen (ST) binding to N-terminal HEAT repeats in the PP2A A subunit induces structural changes transduced to C-terminal HEAT repeats. This enables the C-terminal HEAT repeats in the PP2A A subunit, including HEAT repeat 13, to discriminate between androgen- and androgen antagonist-induced AR conformations. The PP2A-AR interaction was used to show that an AR mutant in prostate cancer cells (T877A) is activated by multiple ligands without acquiring the same conformation as that induced by androgen. The correlation between androgen binding to AR and increased phosphorylation of the activation function 1 (AF-1) region implies that changes in AR conformation or chaperone composition are causal to kinase access to phosphorylation sites. However, AF-1 phosphorylation sites are kinase accessible prior to androgen binding. This suggests that androgens can enhance the phosphorylation state of AR either by negatively regulating the ability of the ligand-binding domain to bind phosphatases or by inducing an AR conformation that is resistant to phosphatase action. SV40 ST subverts this mechanism by promoting the direct transfer of PP2A onto androgen-bound AR, resulting in multisite dephosphorylation.
Figures


References
-
- Andrade, M. A., and P. Bork. 1995. HEAT repeats in the Huntington's disease protein. Nat. Genet. 11:115-116. - PubMed
-
- Chang, C. Y., and D. P. McDonnell. 2002. Evaluation of ligand-dependent changes in AR structure using peptide probes. Mol. Endocrinol. 16:647-660. - PubMed
-
- Chen, F., K. Knecht, C. Leu, S. J. Rutledge, A. Scafonas, C. Gambone, R. Vogel, H. Zhang, V. Kasparcova, C. Bai, S. Harada, A. Schmidt, A. Reszka, and L. Freedman. 2004. Partial agonist/antagonist properties of androstenedione and 4-androsten-3β,17β-diol. J. Steroid Biochem. Mol. Biol. 91:247-257. - PubMed
-
- Cho, U. S., and W. Xu. 2006. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445:53-57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
