Targeting of the ETS factor GABPalpha disrupts neuromuscular junction synaptic function
- PMID: 17325042
- PMCID: PMC1899955
- DOI: 10.1128/MCB.00659-06
Targeting of the ETS factor GABPalpha disrupts neuromuscular junction synaptic function
Abstract
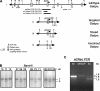
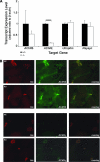
The GA-binding protein (GABP) transcription factor has been shown in vitro to regulate the expression of the neuromuscular proteins utrophin, acetylcholine esterase, and acetylcholine receptor subunits delta and epsilon through the N-box promoter motif (5'-CCGGAA-3'), but its in vivo function remains unknown. A single point mutation within the N-box of the gene encoding the acetylcholine receptor epsilon subunit has been identified in several patients suffering from postsynaptic congenital myasthenic syndrome, implicating the GA-binding protein in neuromuscular function and disease. Since conventional gene targeting results in an embryonic-lethal phenotype, we used conditional targeting to investigate the role of GABPalpha in neuromuscular junction and skeletal muscle development. The diaphragm and soleus muscles from mutant mice display alterations in morphology and distribution of acetylcholine receptor clusters at the neuromuscular junction and neurotransmission properties consistent with reduced receptor function. Furthermore, we confirmed decreased expression of the acetylcholine receptor epsilon subunit and increased expression of the gamma subunit in skeletal muscle tissues. Therefore, the GABP transcription factor aids in the structural formation and function of neuromuscular junctions by regulating the expression of postsynaptic genes.
Figures




References
-
- Brock, J. A., and T. C. Cunnane. 1987. Relationship between the nerve action potential and transmitter release from sympathetic postganglionic nerve terminals. Nature 326:605-607. - PubMed
-
- Chinenov, Y., C. Coombs, and M. E. Martin. 2000. Isolation of a bi-directional promoter directing expression of the mouse GABPα and ATP synthase coupling factor 6 genes. Gene 261:311-320. - PubMed
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
