Second-harmonic imaging microscopy of normal human and keratoconus cornea
- PMID: 17325150
- PMCID: PMC1894888
- DOI: 10.1167/iovs.06-1177
Second-harmonic imaging microscopy of normal human and keratoconus cornea
Abstract
Purpose: The purpose of this study was to evaluate the ability of second-harmonic imaging to identify differences in corneal stromal collagen organization between normal human and keratoconus corneas.
Methods: Six normal corneas from eye bank donors and 13 corneas of patients with keratoconus, obtained after penetrating keratoplasty were examined. A femtosecond titanium-sapphire laser with 800-nm output was used to generate second-harmonic signals collected at 400 nm from central and paracentral corneal tissue blocks. Three-dimensional (3-D) data sets were collected and reconstructed to evaluate the location and orientation of stromal collagen lamellae.
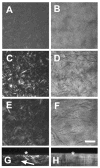
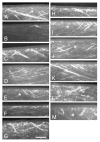
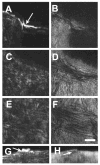
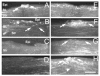
Results: Imaging of second-harmonic signals combined with 3-D reconstruction of the normal cornea identified a high degree of lamellar interweaving, particularly in the anterior cornea. Of note was the detection of lamellae that inserted into Bowman's layer and were oriented transverse to the corneal surface, penetrating posteriorly approximately 120 mum. In keratoconus corneas, imaging second-harmonic signals identified less lamellar interweaving and a marked reduction or loss of lamellae inserting into Bowman's layer in 12 of 13 cases, particularly in regions associated with cone development without breaks in Bowman's layer or scarring.
Conclusions: Compared with normal adult corneas, marked abnormalities were detected in the organization of the anterior corneal collagen lamellae of keratoconus corneas by second harmonic imaging. These structural abnormalities are consistent with the known changes in collagen organization and biomechanical strength of keratoconus.
Figures



References
-
- Campagnola PJ, Loew LM. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat Biotechnol. 2003;21:1356–1360. - PubMed
-
- Hochheimer BF. Second harmonic light generation in the rabbit cornea. Appl Opt. 1982:1516–1518. - PubMed
-
- Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. - PubMed
-
- Teng SW, Tan HY, Peng JL, et al. Multiphoton autofluorescence and second-harmonic generation imaging of the ex vivo porcine eye. Invest Ophthalmol Vis Sci. 2006;47:1216–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

