Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation
- PMID: 17325200
- PMCID: PMC2137910
- DOI: 10.1084/jem.20061792
Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation
Abstract
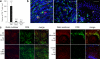
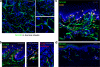
Cytotoxic CD8(+) T cells play a critical role in controlling herpes simplex virus (HSV) infection and reactivation. However, little is known about the spatiotemporal dynamics of CD8(+) T cells during HSV lesion evolution or about their involvement in immune surveillance after lesion resolution. Using quantum dot-conjugated peptide-major histocompatibility complex multimers, we investigated the in vivo localization of HSV-2-specific CD8(+) T cells in sequential biopsies of human genital skin during acute, resolving, and healed stages of HSV-2 reactivation. Our studies revealed that functionally active CD8(+) T cells selectively infiltrated to the site of viral reactivation. After lesion healing in concert with complete reepithelialization and loss of HSV DNA from skin biopsies, HSV-2-specific CD8(+) T cells persisted for more than two months at the dermal-epidermal junction, adjacent to peripheral nerve endings. In two out of the six sequentially studied individuals, HSV-2 DNA reappeared in clinically and histologically normal-appearing skin. Detection of viral DNA was accompanied by increased numbers of both HSV-specific and total CD8(+) T cells in the dermis. These findings indicate that the frequency and clinical course of HSV-2 reactivation in humans is influenced by virus-specific CD8(+) T cells that persist in peripheral mucosa and genital skin after resolution of herpes lesions.
Figures




References
-
- Augenbraun, M., J. Feldman, K. Chirgwin, J. Zenilman, L. Clarke, J. DeHovitz, S. Landesman, and H. Minkoff. 1995. Increased genital shedding of herpes simplex virus type 2 in HIV-seropositive women. Ann. Intern. Med. 123:845–847. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

