Oral presentation bias: a retrospective cohort study
- PMID: 17325393
- PMCID: PMC2652905
- DOI: 10.1136/jech.2006.048603
Oral presentation bias: a retrospective cohort study
Abstract
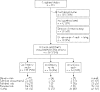
The aim of this paper was to assess oral presentation bias at a national level. This was a retrospective cohort study with initial characteristics of the approved protocols extracted from the committee's archives, and follow-up characteristics obtained from a questionnaire mailed to the principal investigators. A representative sample of French research ethics committees (25/48), the only committees legally endorsed for ethical authorisation in biomedical research, were studied. All completed research protocols, which had been approved in 1994 by these committees, were included. Initial characteristics (design, study size, investigator) of completed studies and follow-up information (direction of results, rates of publication and rates of oral presentation) were collected. Complete information on results and their dissemination was available for 248 completed non-confidential protocols. Half of these (49%) were declared as orally presented. The observed ranking for strategies to disseminate results was the following: orally presented and published, published only, neither orally presented nor published and orally presented only. Confirmatory results were more often orally presented, with an adjusted OR of 6.4 (95% CI 2.69 to 15.22). Other associated variables are the following: national/international scope of the study, protocol writer's university status, adverse events and interim analysis. There is a trend to submit or accept confirmatory results for oral presentations: meetings are a biased representation of research, and oral presentation bias could even be higher than publication bias.
Conflict of interest statement
Competing interests: None.
References
-
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 19902631385–1389. - PubMed
-
- Dickersin K, Min Y I, Meinert C L. Factors influencing publication of research results. Follow‐up of applications submitted to two institutional review boards. JAMA 1992267374–378. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources