The effect of epibatidine on spontaneous and evoked neurotransmitter release in the mouse and guinea pig isolated vas deferens
- PMID: 17325648
- PMCID: PMC2013884
- DOI: 10.1038/sj.bjp.0707183
The effect of epibatidine on spontaneous and evoked neurotransmitter release in the mouse and guinea pig isolated vas deferens
Abstract
Background and purpose: Nicotinic agonists increase sympathetic field-stimulus-evoked contraction of the rodent vas deferens, presumably by increasing evoked neurotransmitter release. This presumption was tested in two species.
Experimental approach: The effect of the nicotinic acetylcholine receptor (nAChR) agonist epibatidine on neurotransmitter release in mouse and guinea pig isolated vas deferens was investigated using contraction studies and conventional intracellular recording techniques.
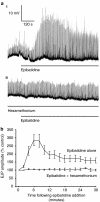
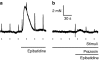
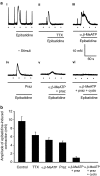
Key results: In 12 of 14 mouse vasa deferentia, slow bath application of epibatidine (100 nM) had no significant effect on excitatory junction potential (EJP) amplitude and spontaneous EJP (SEJP) frequency. However, rapid application of epibatidine to the mouse vas deferens caused an increase in SEJP frequency (by 530%), with no effect on EJP amplitude. Despite the absence of an effect on EJPs, electrically-evoked contractions of the mouse vas deferens were significantly increased in the presence of epibatidine (by 50%). A transient contraction was reliably induced by a higher epibatidine concentration (1 microM). This contraction was significantly reduced in the presence of prazosin, tetrodotoxin, or alpha,beta-methyleneATP. Epibatidine did not induce a contraction in the presence of a combination of prazosin, alpha,beta-methyleneATP and cyclopentolate. In guinea pig vasa deferentia, bath-applied epibatidine potentiated EJP amplitude in a biphasic pattern, lasting for at least 30 minutes.
Conclusion and implications: The nAChR-mediated augmentation of neurogenic contraction is indeed prejunctional, but in the mouse arises from an increase in spontaneous neurotransmitter release that primes smooth muscle for subsequent contraction, while in the guinea pig there is a direct augmentation of evoked neurotransmitter (ATP) release.
Figures

Similar articles
-
Mechanisms involved in nicotinic acetylcholine receptor-induced neurotransmitter release from sympathetic nerve terminals in the mouse vas deferens.PLoS One. 2011;6(12):e29209. doi: 10.1371/journal.pone.0029209. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216213 Free PMC article.
-
Cholinergic innervation of the guinea-pig isolated vas deferens.Naunyn Schmiedebergs Arch Pharmacol. 2007 Dec;376(4):265-74. doi: 10.1007/s00210-007-0198-y. Epub 2007 Nov 9. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17992517 Free PMC article.
-
[Analysis of the contractions evoked by sympathetic nerve stimulation, and thermal effect on the guinea-pig vas deferens--study as a model for the thermotherapy of benign prostatic hyperplasia].Nihon Hinyokika Gakkai Zasshi. 2000 Apr;91(4):459-68. doi: 10.5980/jpnjurol1989.91.459. Nihon Hinyokika Gakkai Zasshi. 2000. PMID: 10826244 Japanese.
-
Cholinergic innervation of the mouse isolated vas deferens.Br J Pharmacol. 2005 Dec;146(7):927-34. doi: 10.1038/sj.bjp.0706357. Br J Pharmacol. 2005. PMID: 16170331 Free PMC article.
-
A1 adenosine receptor modulation of electrically-evoked contractions in the bisected vas deferens and cauda epididymis of the guinea-pig.Br J Pharmacol. 1998 Jul;124(5):964-70. doi: 10.1038/sj.bjp.0701909. Br J Pharmacol. 1998. PMID: 9692782 Free PMC article.
Cited by
-
Dog and human bladders have different neurogenic and nicotinic responses in inner versus outer detrusor muscle layers.Am J Physiol Regul Integr Comp Physiol. 2022 Oct 1;323(4):R589-R600. doi: 10.1152/ajpregu.00084.2022. Epub 2022 Sep 5. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 36062901 Free PMC article.
-
Nerve transfer for restoration of lower motor neuron-lesioned bladder, urethral, and anal sphincter function in a dog model. Part 3. nicotinic receptor characterization.Am J Physiol Regul Integr Comp Physiol. 2023 Oct 1;325(4):R344-R358. doi: 10.1152/ajpregu.00273.2022. Epub 2023 Jul 17. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 37458380 Free PMC article.
-
Dynamic monitoring of NET activity in mature murine sympathetic terminals using a fluorescent substrate.Br J Pharmacol. 2010 Feb;159(4):797-807. doi: 10.1111/j.1476-5381.2009.00574.x. Epub 2010 Feb 5. Br J Pharmacol. 2010. PMID: 20136837 Free PMC article.
-
Mechanisms involved in nicotinic acetylcholine receptor-induced neurotransmitter release from sympathetic nerve terminals in the mouse vas deferens.PLoS One. 2011;6(12):e29209. doi: 10.1371/journal.pone.0029209. Epub 2011 Dec 22. PLoS One. 2011. PMID: 22216213 Free PMC article.
References
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. - PubMed
-
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol. 1994;45:563–569. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Brain KL, Trout SJ, Jackson VM, Dass N, Cunnane TC. Nicotine induces calcium spikes in single nerve terminal varicosities: a role for intracellular calcium stores. Neuroscience. 2001;106:395–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

