Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors
- PMID: 17325663
- PMCID: PMC2084378
- DOI: 10.1038/sj.onc.1210329
Epigenetic inactivation of the tissue inhibitor of metalloproteinase-2 (TIMP-2) gene in human prostate tumors
Abstract
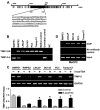
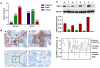
Gene silencing via CpG island methylation in the promoter region is one of the mechanisms by which tumor suppressor genes are inactivated in human cancers. Previous studies have shown that the tissue inhibitors of metalloproteinases (TIMP)-2 gene, which is an endogenous inhibitor of matrix metalloproteinases involved in cell invasion and tumorigenesis, is downregulated or silenced in a variety of human cancer cell lines. Here, we investigated the mechanism underlying TIMP-2 expression in prostate cancer cell lines and primary prostate tumor samples. We observed a strong correlation between promoter hypermethylation and lost expression of TIMP-2 gene, which was supported by other results demonstrating that promoter demethylation by 5-aza-2'-deoxycytidine and trichostatin A reactivated TIMP-2 and restored its expression in TIMP-2-silenced metastatic prostate cell lines. These results were further substantiated by a chromatin immunoprecipitation assay, showing the preferential binding of MeCP2 to methylated CpG island in TIMP-2-silenced metastatic prostate cell lines. In vitro Matrigel invasion assays showed that re-expression of TIMP-2 after a combined treatment with 5-aza and trichostatin-A in metastatic prostate cells resulted in a significant reduction of tumor cell invasion. Furthermore, CpG methylation of TIMP-2 promoter was also shown in primary prostate tumors that expressed decreased TIMP-2 protein levels. These results suggest that the downregulation of the TIMP-2 gene is associated with promoter methylation and that this may play an important role in prostate cancer progression during the invasive and metastatic stages of the disease.
Figures




Similar articles
-
CpG island promoter methylation and silencing of 14-3-3sigma gene expression in LNCaP and Tramp-C1 prostate cancer cell lines is associated with methyl-CpG-binding protein MBD2.Oncogene. 2006 Aug 3;25(33):4559-72. doi: 10.1038/sj.onc.1209462. Epub 2006 Jun 19. Oncogene. 2006. PMID: 16786000 Free PMC article.
-
Epigenetic inactivation of Betaig-h3 gene in human cancer cells.Cancer Res. 2006 May 1;66(9):4566-73. doi: 10.1158/0008-5472.CAN-05-2130. Cancer Res. 2006. PMID: 16651406
-
DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation.Prostaglandins Other Lipid Mediat. 2007 Jan;82(1-4):185-97. doi: 10.1016/j.prostaglandins.2006.05.015. Epub 2006 Jul 11. Prostaglandins Other Lipid Mediat. 2007. PMID: 17164146
-
Tissue inhibitor of matrix metalloproteinase-3 has both anti-metastatic and anti-tumourigenic properties.Clin Exp Metastasis. 2020 Feb;37(1):69-76. doi: 10.1007/s10585-019-10017-y. Epub 2020 Jan 1. Clin Exp Metastasis. 2020. PMID: 31894441 Review.
-
Promoter hypermethylation in prostate cancer.Cancer Control. 2010 Oct;17(4):245-55. doi: 10.1177/107327481001700405. Cancer Control. 2010. PMID: 20861812 Free PMC article. Review.
Cited by
-
Modeling human prostate cancer progression in vitro.Carcinogenesis. 2019 Jul 20;40(7):893-902. doi: 10.1093/carcin/bgy185. Carcinogenesis. 2019. PMID: 30590461 Free PMC article.
-
Differential gene methylation patterns in cancerous and non‑cancerous cells.Oncol Rep. 2019 Jul;42(1):43-54. doi: 10.3892/or.2019.7159. Epub 2019 May 15. Oncol Rep. 2019. PMID: 31115550 Free PMC article.
-
Epigenetic control of the invasion-promoting MT1-MMP/MMP-2/TIMP-2 axis in cancer cells.J Biol Chem. 2009 May 8;284(19):12727-34. doi: 10.1074/jbc.M900273200. Epub 2009 Mar 13. J Biol Chem. 2009. PMID: 19286653 Free PMC article.
-
Epigenetic regulation of matrix metalloproteinases and their collagen substrates in cancer.Biomol Concepts. 2011 Jun;2(3):135-147. doi: 10.1515/BMC.2011.017. Biomol Concepts. 2011. PMID: 21779312 Free PMC article.
-
Promoter methylation in prostate cancer and its application for the early detection of prostate cancer using serum and urine samples.Biomark Cancer. 2010 Feb 18;2:17-33. doi: 10.4137/BIC.S3187. Biomark Cancer. 2010. PMID: 24179382 Free PMC article. Review.
References
-
- Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798–802. - PubMed
-
- Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. - PubMed
-
- DeClerck YA, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992;52:701–708. - PubMed
-
- Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61:3225–3229. - PubMed
-
- Galm O, Suzuki H, Akiyama Y, Esteller M, Brock MV, Osieka R, et al. Inactivation of the tissue inhibitor of metalloproteinases-2 gene by promoter hypermethylation in lymphoid malignancies. Oncogene. 2005;24:4799–4805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

