Impact of stroma on the growth, microcirculation, and metabolism of experimental prostate tumors
- PMID: 17325744
- PMCID: PMC1803035
- DOI: 10.1593/neo.06688
Impact of stroma on the growth, microcirculation, and metabolism of experimental prostate tumors
Abstract
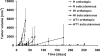
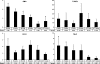
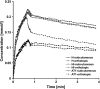
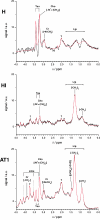
In prostate cancers (PCa), the formation of malignant stroma may substantially influence tumor phenotype and aggressiveness. Thus, the impact of the orthotopic and subcutaneous implantations of hormone-sensitive (H), hormone-insensitive (HI), and anaplastic (AT1) Dunning PCa in rats on growth, microcirculation, and metabolism was investigated. For this purpose, dynamic contrast-enhanced magnetic resonance imaging and (1)H magnetic resonance spectroscopy ([(1)H]MRS) were applied in combination with histology. Consistent observations revealed that orthotopic H tumors grew significantly slower compared to subcutaneous ones, whereas the growth of HI and AT1 tumors was comparable at both locations. Histologic analysis indicated that glandular differentiation and a close interaction of tumor cells and smooth muscle cells (SMC) were associated with slow tumor growth. Furthermore, there was a significantly lower SMC density in subcutaneous H tumors than in orthotopic H tumors. Perfusion was observed to be significantly lower in orthotopic H tumors than in subcutaneous H tumors. Regional blood volume and permeability-surface area product showed no significant differences between tumor models and their implantation sites. Differences in growth between subcutaneous and orthotopic H tumors can be attributed to tumor-stroma interaction and perfusion. Here, SMC, may stabilize glandular structures and contribute to the maintenance of differentiated phenotype.
Figures



References
-
- Rich AR. On the frequency of occurrence of occult carcinoma of the prostate. J Urol. 1935;33:215–223.
-
- Yatani R, Chigusa I, Akazaki K, Stemmermann GN, Welsh RA, Correa P. Geographic pathology of latent prostatic carcinoma. Int J Cancer. 1982;29:611–616. - PubMed
-
- Silverberg E, Lubera JA. Cancer statistics 1989. CA Cancer J Clin. 1989;39:3–30. - PubMed
-
- Etzioni R, Penson DF, Legler LM, di Tommaso D, Boer R, Gann PH, Feuer EJ. Overdiagnosis due to prostate-specific-antigen screening: lesson from US prostate cancer incidence trend. J Natl Cancer Inst. 2002;94:981–990. - PubMed
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
