Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation therapy
- PMID: 17325745
- PMCID: PMC1803036
- DOI: 10.1593/neo.06739
Androgen induces adaptation to oxidative stress in prostate cancer: implications for treatment with radiation therapy
Abstract
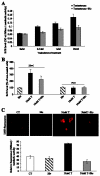
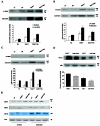
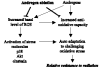
Radiation therapy is a standard treatment for prostate cancer (PC). The postulated mechanism of action for radiation therapy is the generation of reactive oxygen species (ROS). Adjuvant androgen deprivation (AD) therapy has been shown to confer a survival advantage over radiation alone in high-risk localized PC. However, the mechanism of this interaction is unclear. We hypothesize that androgens modify the radioresponsiveness of PC through the regulation of cellular oxidative homeostasis. Using androgen receptor (AR)(+) 22rv1 and AR(-) PC3 human PC cell lines, we demonstrated that testosterone increased basal reactive oxygen species (bROS) levels, resulting in dose-dependent activation of phospho-p38 and pAKT, and increased expression of clusterin, catalase, and manganese superoxide dismutase. Similar data were obtained in three human PC xenografts; WISH-PC14, WISH-PC23, and CWR22, growing in testosterone-supplemented or castrated SCID mice. These effects were reversible through AD or through incubation with a reducing agent. Moreover, testosterone increased the activity of catalase, superoxide dismutases, and glutathione reductase. Consequently, AD significantly facilitated the response of AR(+) cells to oxidative stress challenge. Thus, testosterone induces a preset cellular adaptation to radiation through the generation of elevated bROS, which is modified by AD. These findings provide a rational for combined hormonal and radiation therapy for localized PC.
Figures





Similar articles
-
Inter-related in vitro effects of androgens, fatty acids and oxidative stress in prostate cancer: a mechanistic model supporting prevention strategies.Int J Oncol. 2010 Oct;37(4):761-6. doi: 10.3892/ijo_00000725. Int J Oncol. 2010. PMID: 20811696
-
Endostatin inhibits androgen-independent prostate cancer growth by suppressing nuclear receptor-mediated oxidative stress.FASEB J. 2017 Apr;31(4):1608-1619. doi: 10.1096/fj.201601178R. Epub 2017 Jan 9. FASEB J. 2017. PMID: 28069826 Free PMC article.
-
Androgens induce oxidative stress and radiation resistance in prostate cancer cells though NADPH oxidase.Prostate Cancer Prostatic Dis. 2010 Mar;13(1):39-46. doi: 10.1038/pcan.2009.24. Epub 2009 Jun 23. Prostate Cancer Prostatic Dis. 2010. PMID: 19546883 Free PMC article.
-
Inhibition of the Akt, cyclooxygenase-2, and matrix metalloproteinase-9 pathways in combination with androgen deprivation therapy: potential therapeutic approaches for prostate cancer.Mol Carcinog. 2005 Sep;44(1):1-10. doi: 10.1002/mc.20121. Mol Carcinog. 2005. PMID: 16044418 Review.
-
Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer.Free Radic Biol Med. 2011 Oct 1;51(7):1320-8. doi: 10.1016/j.freeradbiomed.2011.07.011. Epub 2011 Jul 23. Free Radic Biol Med. 2011. PMID: 21820046 Review.
Cited by
-
Associations of LIG4 and HSPB1 genetic polymorphisms with risk of radiation-induced lung injury in lung cancer patients treated with radiotherapy.Biomed Res Int. 2015;2015:860373. doi: 10.1155/2015/860373. Epub 2015 Feb 25. Biomed Res Int. 2015. PMID: 25811031 Free PMC article.
-
Xanthine Oxidase/Dehydrogenase Activity as a Source of Oxidative Stress in Prostate Cancer Tissue.Diagnostics (Basel). 2020 Sep 3;10(9):668. doi: 10.3390/diagnostics10090668. Diagnostics (Basel). 2020. PMID: 32899343 Free PMC article.
-
The Androgen Hormone-Induced Increase in Androgen Receptor Protein Expression Is Caused by the Autoinduction of the Androgen Receptor Translational Activity.Curr Issues Mol Biol. 2022 Jan 25;44(2):597-608. doi: 10.3390/cimb44020041. Curr Issues Mol Biol. 2022. PMID: 35723327 Free PMC article.
-
Dehydroepiandrosterone-induces miR-21 transcription in HepG2 cells through estrogen receptor β and androgen receptor.Mol Cell Endocrinol. 2014 Jul 5;392(1-2):23-36. doi: 10.1016/j.mce.2014.05.007. Epub 2014 May 17. Mol Cell Endocrinol. 2014. PMID: 24845419 Free PMC article.
-
Lentivirus-mediated Nox4 shRNA invasion and angiogenesis and enhances radiosensitivity in human glioblastoma.Oxid Med Cell Longev. 2014;2014:581732. doi: 10.1155/2014/581732. Epub 2014 Apr 27. Oxid Med Cell Longev. 2014. PMID: 24868315 Free PMC article.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Nichol A, Chung P, Lockwood G, Rosewall T, Divanbiegi L, Sweet J, Toi A, Bayley A, Bristow R, Crook J, et al. A phase II study of localized prostate cancer treated to 75.6 Gy with 3D conformal radiotherapy. Radiother Oncol. 2005;76:11–17. - PubMed
-
- Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, Reuter VE, Venkatraman ES, Leibel SA. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. - PubMed
-
- Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E, von Eschenbach AC, Kuban DA, Rosen I. Prostate cancer radiation dose response: results of the M.D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. - PubMed
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomized trial. Lancet. 2002;360:103–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
