Intermediates in dioxygen activation by methane monooxygenase: a QM/MM study
- PMID: 17326634
- PMCID: PMC2517126
- DOI: 10.1021/ja0654074
Intermediates in dioxygen activation by methane monooxygenase: a QM/MM study
Abstract
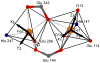
Protein effects in the activation of dioxygen by methane monooxygenase (MMO) were investigated by using combined QM/MM and broken-symmetry Density Functional Theory (DFT) methods. The effects of a novel empirical scheme recently developed by our group on the relative DFT energies of the various intermediates in the catalytic cycle are investigated. Inclusion of the protein leads to much better agreement between the experimental and computed geometric structures for the reduced form (MMOH(red)). Analysis of the electronic structure of MMOH(red) reveals that the two iron atoms have distinct environments. Different coordination geometries tested for the MMOH(peroxo) intermediate reveal that, in the protein environment, the mu-eta2,eta2 structure is more stable than the others. Our analysis also shows that the protein helps to drive reactants toward products along the reaction path. Furthermore, these results demonstrate the importance of including the protein environment in our models and the usefulness of the QM/MM approach for accurate modeling of enzymatic reactions. A discrepancy remains in our calculation of the Fe-Fe distance in our model of HQ as compared to EXAFS data obtained several years ago, for which we currently do not have an explanation.
Figures




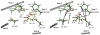

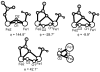




Similar articles
-
Structure, electronic configuration, and Mössbauer spectral parameters of an antiferromagnetic Fe2-peroxo intermediate of methane monooxygenase.Dalton Trans. 2012 Jan 21;41(3):995-1003. doi: 10.1039/c1dt11656h. Epub 2011 Nov 21. Dalton Trans. 2012. PMID: 22101614
-
Structural model studies for the peroxo intermediate P and the reaction pathway from P-->Q of methane monooxygenase using broken-symmetry density functional calculations.Inorg Chem. 2008 Apr 21;47(8):2975-86. doi: 10.1021/ic701194b. Inorg Chem. 2008. PMID: 18366153
-
Dioxygen activation in methane monooxygenase: a theoretical study.J Am Chem Soc. 2004 Mar 10;126(9):2978-90. doi: 10.1021/ja036506+. J Am Chem Soc. 2004. PMID: 14995216
-
Dioxygen activation in soluble methane monooxygenase.Acc Chem Res. 2011 Apr 19;44(4):280-8. doi: 10.1021/ar1001473. Epub 2011 Mar 10. Acc Chem Res. 2011. PMID: 21391602 Free PMC article. Review.
-
[Analysis of enzymatic reactions by quantum chemical calculations].Yakugaku Zasshi. 2012;132(8):863-71. doi: 10.1248/yakushi.132.863. Yakugaku Zasshi. 2012. PMID: 22864343 Review. Japanese.
Cited by
-
Enzymatic oxidation of methane.Biochemistry. 2015 Apr 14;54(14):2283-94. doi: 10.1021/acs.biochem.5b00198. Epub 2015 Apr 1. Biochemistry. 2015. PMID: 25806595 Free PMC article. Review.
-
Structure-Spectroscopy Correlations for Intermediate Q of Soluble Methane Monooxygenase: Insights from QM/MM Calculations.J Am Chem Soc. 2021 May 5;143(17):6560-6577. doi: 10.1021/jacs.1c01180. Epub 2021 Apr 22. J Am Chem Soc. 2021. PMID: 33884874 Free PMC article.
-
2-Phenoxypyridyl dinucleating ligands for assembly of diiron(II) complexes: efficient reactivity with O(2) to form (mu-Oxo)diiron(III) units.Inorg Chem. 2009 Nov 16;48(22):10708-19. doi: 10.1021/ic901711c. Inorg Chem. 2009. PMID: 19845332 Free PMC article.
-
Insights into the different dioxygen activation pathways of methane and toluene monooxygenase hydroxylases.J Am Chem Soc. 2011 May 18;133(19):7384-97. doi: 10.1021/ja110287y. Epub 2011 Apr 25. J Am Chem Soc. 2011. PMID: 21517016 Free PMC article.
-
Oxidative photosynthetic water splitting: energetics, kinetics and mechanism.Photosynth Res. 2007 Jun;92(3):407-25. doi: 10.1007/s11120-007-9185-x. Epub 2007 Jul 24. Photosynth Res. 2007. PMID: 17647091 Review.
References
-
- Feig AL, Lippard SJ. Chem Rev. 1994;94:759.
-
- Liu KE, Lippard SJ. Adv Inorg Chem. 1995;42:263.
-
- Wallar BJ, Lipscomb JD. Chem Rev. 1996;96:2625. - PubMed
-
- Valentine AM, Lippard SJ. J Chem Soc Dalton Trans. 1997:3925.
-
- Deeth RJ, Dalton H. J Biol Inorg Chem. 1998;3:302.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

