When should potentially false research findings be considered acceptable?
- PMID: 17326703
- PMCID: PMC1808081
- DOI: 10.1371/journal.pmed.0040026
When should potentially false research findings be considered acceptable?
Abstract
Ioannidis estimated that most published research findings are false, but he did not indicate when, if at all, potentially false research results may be considered as acceptable to society. We combined our two previously published models to calculate the probability above which research findings may become acceptable. A new model indicates that the probability above which research results should be accepted depends on the expected payback from the research (the benefits) and the inadvertent consequences (the harms). This probability may dramatically change depending on our willingness to tolerate error in accepting false research findings. Our acceptance of research findings changes as a function of what we call "acceptable regret," i.e., our tolerance of making a wrong decision in accepting the research hypothesis. We illustrate our findings by providing a new framework for early stopping rules in clinical research (i.e., when should we accept early findings from a clinical trial indicating the benefits as true?). Obtaining absolute "truth" in research is impossible, and so society has to decide when less-than-perfect results may become acceptable.
Conflict of interest statement
Figures
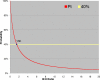
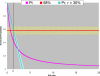
References
-
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. - DOI - PMC - PubMed
-
- Djulbegovic B, Hozo I, Schwartz A, McMasters K. Acceptable regret in medical decision making. Med Hypotheses. 1999;53:253–259. - PubMed
-
- Djulbegovic B, Hozo I. At what degree of belief in a research hypothesis is a trial in humans justified? J Eval Clin Practice. 2002;8:269–276. - PubMed
-
- Pauker S, Kassirer J. Therapeutic decision making: A cost benefit analysis. N Engl J Med. 1975;293:229–234. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

