Ets-2 and C/EBP-beta are important mediators of ovine trophoblast Kunitz domain protein-1 gene expression in trophoblast
- PMID: 17326832
- PMCID: PMC1817651
- DOI: 10.1186/1471-2199-8-14
Ets-2 and C/EBP-beta are important mediators of ovine trophoblast Kunitz domain protein-1 gene expression in trophoblast
Abstract
Background: The trophoblast Kunitz domain proteins (TKDPs) constitute a highly expressed, placenta-specific, multigene family restricted to ruminant ungulates and characterized by a C-terminal "Kunitz" domain, preceded by one or more unique N-terminal domains. TKDP-1 shares an almost identical expression pattern with interferon-tau, the "maternal recognition of pregnancy protein" in ruminants. Our goal here has been to determine whether the ovine (ov) Tkdp-1 and IFNT genes possess a similar transcriptional code.
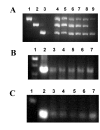
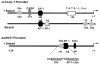
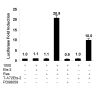
Results: The ovTkdp-1 promoter has been cloned and characterized. As with the IFNT promoter, the Tkdp-1 promoter is responsive to Ets-2, and promoter-driven reporter activity can be increased over 700-fold in response to over-expression of Ets-2 and a constitutively active form of protein Kinase A (PKA). Unexpectedly, the promoter element of Tkdp-1 responsible for this up-regulation, unlike that of the IFNT, does not bind Ets-2. However, mutation of a CCAAT/enhancer binding element within this control region not only reduced basal transcriptional activity, but prevented Ets-2 as well as cyclic adenosine 5'-monophosphate (cAMP)/PKA and Ras/mitogen-activated protein kinase (MAPK) responsiveness. In vitro binding experiments and in vivo protein-protein interaction assays implicated CCAAT/enhancer binding protein-beta (C/EBP-beta) as involved in up-regulating the Tkdp-1 promoter activity. A combination of Ets-2 and C/EBP-beta can up-regulate expression of the minimal Tkdp-1 promoter as much as 930-fold in presence of a cAMP analog. An AP-1-like element adjacent to the CCAAT enhancer, which binds Jun family members, is required for basal and cAMP/ C/EBP-beta-dependent activation of the gene, but not for Ets-2-dependent activity.
Conclusion: This paper demonstrates how Ets-2, a key transcription factor for trophoblast differentiation and function, can control expression of two genes (Tkdp-1 and IFNT) having similar spatial and temporal expression patterns via very different mechanisms.
Figures
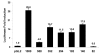
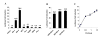
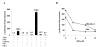
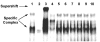
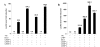
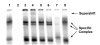
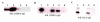
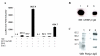
Similar articles
-
Combinatorial roles of protein kinase A, Ets2, and 3',5'-cyclic-adenosine monophosphate response element-binding protein-binding protein/p300 in the transcriptional control of interferon-tau expression in a trophoblast cell line.Mol Endocrinol. 2008 Feb;22(2):331-43. doi: 10.1210/me.2007-0300. Epub 2007 Nov 1. Mol Endocrinol. 2008. PMID: 17975022 Free PMC article.
-
Regulation of interferon-tau (IFN-tau) gene promoters by growth factors that target the Ets-2 composite enhancer: a possible model for maternal control of IFN-tau production by the conceptus during early pregnancy.Endocrinology. 2004 Oct;145(10):4452-60. doi: 10.1210/en.2004-0606. Epub 2004 Jun 24. Endocrinology. 2004. PMID: 15217985
-
Coactivator CBP in the regulation of conceptus IFNtau gene transcription.Mol Reprod Dev. 2003 May;65(1):23-9. doi: 10.1002/mrd.10293. Mol Reprod Dev. 2003. PMID: 12658630
-
Evolution of the interferon tau genes and their promoters, and maternal-trophoblast interactions in control of their expression.Reprod Suppl. 2003;61:239-51. Reprod Suppl. 2003. PMID: 14635939 Review.
-
Transcriptional control of IFNT expression.Reproduction. 2017 Nov;154(5):F21-F31. doi: 10.1530/REP-17-0330. Reproduction. 2017. PMID: 28982936 Free PMC article. Review.
Cited by
-
Fine-scale quantification of HCG beta gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues.Mol Hum Reprod. 2008 Jan;14(1):23-31. doi: 10.1093/molehr/gam082. Epub 2007 Nov 29. Mol Hum Reprod. 2008. PMID: 18048458 Free PMC article.
-
A hypomorphic vasopressin allele prevents anxiety-related behavior.PLoS One. 2009;4(4):e5129. doi: 10.1371/journal.pone.0005129. Epub 2009 Apr 9. PLoS One. 2009. PMID: 19357765 Free PMC article.
-
Artemisinin selectively decreases functional levels of estrogen receptor-alpha and ablates estrogen-induced proliferation in human breast cancer cells.Carcinogenesis. 2008 Dec;29(12):2252-8. doi: 10.1093/carcin/bgn214. Epub 2008 Sep 10. Carcinogenesis. 2008. PMID: 18784357 Free PMC article.
-
Ets-2 is involved in transcriptional regulation of C1qTNF-related protein 5 in muscle cells.Mol Biol Rep. 2012 Oct;39(10):9445-51. doi: 10.1007/s11033-012-1809-3. Epub 2012 Jun 28. Mol Biol Rep. 2012. PMID: 22740135
-
A genome resource to address mechanisms of developmental programming: determination of the fetal sheep heart transcriptome.J Physiol. 2012 Jun 15;590(12):2873-84. doi: 10.1113/jphysiol.2011.222398. Epub 2012 Apr 16. J Physiol. 2012. PMID: 22508961 Free PMC article.
References
-
- Schweitz H, Heurteaux C, Bois P, Moinier D, Romey G, Lazdunski M. Calcicludine, a Venom Peptide of the Kunitz-type Protease Inhibitor Family, is a Potent blocker of high-threshold Ca+2 Channels with a High-affinity for L-type channels in cerebellar Granule Neurons. Proc Natl Acad Sci USA. 1994;91:878–882. doi: 10.1073/pnas.91.3.878. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

