Role of aggregation conditions in structure, stability, and toxicity of intermediates in the Abeta fibril formation pathway
- PMID: 17327396
- PMCID: PMC2203338
- DOI: 10.1110/ps.062514807
Role of aggregation conditions in structure, stability, and toxicity of intermediates in the Abeta fibril formation pathway
Abstract
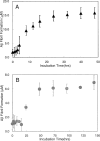
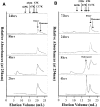
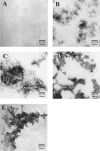
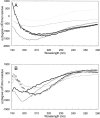
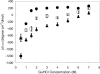
beta-amyloid peptide (Abeta) is one of the main protein components of senile plaques associated with Alzheimer's disease (AD). Abeta readily aggregates to forms fibrils and other aggregated species that have been shown to be toxic in a number of studies. In particular, soluble oligomeric forms are closely related to neurotoxicity. However, the relationship between neurotoxicity and the size of Abeta aggregates or oligomers is still under investigation. In this article, we show that different Abeta incubation conditions in vitro can affect the rate of Abeta fibril formation, the conformation and stability of intermediates in the aggregation pathway, and toxicity of aggregated species formed. When gently agitated, Abeta aggregates faster than Abeta prepared under quiescent conditions, forming fibrils. The morphology of fibrils formed at the end of aggregation with or without agitation, as observed in electron micrographs, is somewhat different. Interestingly, intermediates or oligomers formed during Abeta aggregation differ greatly under agitated and quiescent conditions. Unfolding studies in guanidine hydrochloride indicate that fibrils formed under quiescent conditions are more stable to unfolding in detergent than aggregation associated oligomers or Abeta fibrils formed with agitation. In addition, Abeta fibrils formed under quiescent conditions were less toxic to differentiated SH-SY5Y cells than the Abeta aggregation associated oligomers or fibrils formed with agitation. These results highlight differences between Abeta aggregation intermediates formed under different conditions and provide insight into the structure and stability of toxic Abeta oligomers.
Figures
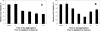
References
-
- Bitan G. and Teplow, D.B. 2004. Rapid photochemical cross-linking—A new tool for studies of metastable, amyloidogenic protein assemblies. Acc. Chem. Res. 37: 357–364. - PubMed
-
- Chromy B.A., Nowak, R.J., Lambert, M.P., Viola, K.L., Chang, L., Velasco, P.T., Jones, B.W., Fernandez, S.J., Lacor, P.N., Horowitz, P., et al. 2003. Self-assembly of Aβ(1-42) into globular neurotoxins. Biochemistry 42: 12749–12760. - PubMed
-
- Dahlgren K.N., Manelli, A.M., Stine Jr, W.B., Baker, L.K., Krafft, G.A., and LaDu, M.J. 2002. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 277: 32046–32053. - PubMed
-
- Demuro A., Mina, E., Kayed, R., Milton, S.C., Parker, I., and Glabe, C.G. 2005. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 280: 17294–17300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

