Mannan-mediated gene delivery for cancer immunotherapy
- PMID: 17328786
- PMCID: PMC2265888
- DOI: 10.1111/j.1365-2567.2006.02506.x
Mannan-mediated gene delivery for cancer immunotherapy
Abstract
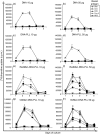
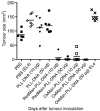
Recent years have seen a resurgence in interest in the development of efficient non-viral delivery systems for DNA vaccines and gene therapy. We have previously used oxidized and reduced mannan as carriers for protein delivery to antigen-presenting cells by targeting the receptors that bind mannose, resulting in efficient induction of cellular responses. In the present study, oxidized mannan and reduced mannan were used as receptor-mediated gene transfer ligands for cancer immunotherapy. In vivo studies in C57BL/6 mice showed that injection of DNA encoding ovalbumin (OVA) complexed to oxidized or reduced mannan-poly-L-lysine induced CD8 and CD4 T-cell responses as well as antibody responses leading to protection of mice from OVA+ tumours. Both oxidized and reduced mannan delivery was superior to DNA alone or DNA-poly-L-lysine. These studies demonstrate the potential of oxidized and reduced mannan for efficient receptor-mediated gene delivery in vivo, particularly as DNA vaccines for cancer immunotherapy.
Figures

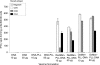

Similar articles
-
Oxidized and reduced mannan mediated MUC1 DNA immunization induce effective anti-tumor responses.Vaccine. 2008 Jul 23;26(31):3827-34. doi: 10.1016/j.vaccine.2008.05.008. Epub 2008 May 22. Vaccine. 2008. PMID: 18550230
-
Poly-L-lysine-coated nanoparticles: a potent delivery system to enhance DNA vaccine efficacy.Vaccine. 2007 Jan 26;25(7):1316-27. doi: 10.1016/j.vaccine.2006.09.086. Epub 2006 Oct 10. Vaccine. 2007. PMID: 17052812
-
Activation of antigen-specific T cell-responses by mannan-decorated PLGA nanoparticles.Pharm Res. 2011 Sep;28(9):2288-301. doi: 10.1007/s11095-011-0459-9. Epub 2011 May 11. Pharm Res. 2011. PMID: 21560020
-
Applications of mannose-binding lectins and mannan glycoconjugates in nanomedicine.J Nanopart Res. 2022;24(11):228. doi: 10.1007/s11051-022-05594-1. Epub 2022 Nov 4. J Nanopart Res. 2022. PMID: 36373057 Free PMC article. Review.
-
Receptor-mediated DNA delivery approaches to human gene therapy.Biologicals. 1995 Mar;23(1):13-6. doi: 10.1016/1045-1056(95)90004-7. Biologicals. 1995. PMID: 7619428 Review.
Cited by
-
N-glycan targeted gene delivery to the dendritic cell SIGN receptor.Bioconjug Chem. 2010 Aug 18;21(8):1479-85. doi: 10.1021/bc1000824. Bioconjug Chem. 2010. PMID: 20715853 Free PMC article.
-
Dynamic Imine Bonding Facilitates Mannan Release from a Nanofibrous Peptide Hydrogel.Bioconjug Chem. 2023 Jan 18;34(1):193-203. doi: 10.1021/acs.bioconjchem.2c00461. Epub 2022 Dec 29. Bioconjug Chem. 2023. PMID: 36580277 Free PMC article.
-
A multivalent mRNA vaccine elicits robust immune responses and confers protection in a murine model of monkeypox virus infection.Nat Commun. 2025 Aug 9;16(1):7373. doi: 10.1038/s41467-025-61699-w. Nat Commun. 2025. PMID: 40783493 Free PMC article.
-
The Long Road of Immunotherapeutics against Multiple Sclerosis.Brain Sci. 2020 May 11;10(5):288. doi: 10.3390/brainsci10050288. Brain Sci. 2020. PMID: 32403377 Free PMC article.
-
Fabrication of a novel core-shell gene delivery system based on a brush-like polycation of alpha, beta-poly (L-aspartate-graft-PEI).Pharm Res. 2009 Sep;26(9):2152-63. doi: 10.1007/s11095-009-9928-9. Epub 2009 Jun 26. Pharm Res. 2009. PMID: 19557504
References
-
- Kumar VV, Singh RS, Chaudhuri A. Cationic transfection lipids in gene therapy: successes, set-backs, challenges and promises. Curr Med Chem. 2003;10:1297–306. - PubMed
-
- Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721–34. - PubMed
-
- Cui Z, Mumper RJ. Microparticles and nanoparticles as delivery systems for DNA vaccines. Crit Rev Ther Drug Carrier Syst. 2003;20:103–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

