Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene
- PMID: 17328801
- PMCID: PMC1808448
- DOI: 10.1186/1742-2094-4-9
Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene
Abstract
Background: Microglial neuroinflammation is thought to play a role in the pathogenesis of amyotrophic lateral sclerosis (ALS). The purpose of this study was to provide a histopathological evaluation of the microglial neuroinflammatory response in a rodent model of ALS, the SOD1G93A transgenic rat.
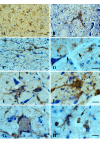
Methods: Multiple levels of the CNS from spinal cord to cerebral cortex were studied in SOD1G93A transgenic rats during three stages of natural disease progression, including presymptomatic, early symptomatic (onset), and late symptomatic (end stage), using immuno- and lectin histochemical markers for microglia, such as OX-42, OX-6, and Griffonia simplicifolia isolectin B4.
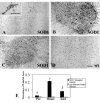
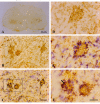
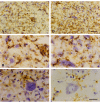
Results: Our studies revealed abnormal aggregates of microglia forming in the spinal cord as early as the presymptomatic stage. During the symptomatic stages there was prominent formation of multinucleated giant cells through fusion of microglial cells in the spinal cord, brainstem, and red nucleus of the midbrain. Other brain regions, including substantia nigra, cranial nerve nuclei, hippocampus and cortex showed normal appearing microglia. In animals during end stage disease at 4-5 months of age virtually all microglia in the spinal cord gray matter showed extensive fragmentation of their cytoplasm (cytorrhexis), indicative of widespread microglial degeneration. Few microglia exhibiting nuclear fragmentation (karyorrhexis) indicative of apoptosis were identified at any stage.
Conclusion: The current findings demonstrate the occurrence of severe abnormalities in microglia, such as cell fusions and cytorrhexis, which may be the result of expression of mutant SOD1 in these cells. The microglial changes observed are different from those that accompany normal microglial activation, and they demonstrate that aberrant activation and degeneration of microglia is part of the pathogenesis of motor neuron disease.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

