Antiviral antibodies are necessary for control of simian immunodeficiency virus replication
- PMID: 17329327
- PMCID: PMC1900210
- DOI: 10.1128/JVI.02444-06
Antiviral antibodies are necessary for control of simian immunodeficiency virus replication
Abstract
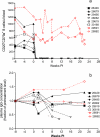
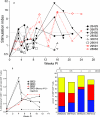
To better define the role of B cells in the control of pathogenic simian immunodeficiency virus (SIV) replication, six rhesus monkeys were depleted of B cells by intravenous infusion of rituximab (anti-CD20) 28 days and 7 days before intravaginal SIVmac239 inoculation and every 21 days thereafter until AIDS developed. Although the blood and tissues were similarly depleted of B cells, anti-SIV immunoglobulin G (IgG) antibody responses were completely blocked in only three of the six animals. In all six animals, levels of viral RNA (vRNA) in plasma peaked at 2 weeks and declined by 4 weeks postinoculation (PI). However, the three animals prevented from making an anti-SIV antibody response had significantly higher plasma vRNA levels through 12 weeks PI (P = 0.012). The remaining three B-cell-depleted animals made moderate anti-SIV IgG antibody responses, maintained moderate plasma SIV loads, and showed an expected rate of disease progression, surviving to 24 weeks PI without developing AIDS. In contrast, all three of the B-cell-depleted animals prevented from making anti-SIV IgG responses developed AIDS by 16 weeks PI (P = 0.0001). These observations indicate that antiviral antibody responses are critical in maintaining effective control of SIV replication at early time points postinfection.
Figures




References
-
- Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. - PMC - PubMed
-
- Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 25:456-467. - PubMed
-
- Dailey, P. J., M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 24:209.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

