Germination ecophysiology of Annona crassiflora seeds
- PMID: 17329406
- PMCID: PMC2802908
- DOI: 10.1093/aob/mcm016
Germination ecophysiology of Annona crassiflora seeds
Abstract
Background and aims: Little is known about environmental factors that break morphophysiological dormancy in seeds of the Annonaceae and the mechanisms involved. The aim of this study was to characterize the morphological and physiological components of dormancy of Annona crassiflora, a tree species native to the Cerrado of Brazil, in an ecophysiological context.
Methods: Morphological and biochemical characteristics of both embryo and endosperm were monitored during dormancy break and germination at field conditions. Seeds were buried in the field and exhumed monthly for 2 years. Germination, embryo length and endosperm digestion, with endo-beta-mannanase activity as a marker, were measured in exhumed seeds, and scanning electron microscopy was used to detect cell division. The effect of constant low and high temperatures and exogenous gibberellins on dormancy break and germination was also tested under laboratory conditions.
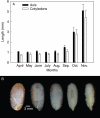
Key results: After burial in April, A. crassiflora seeds lost their physiological dormancy in the winter months with lowest monthly average minimum temperatures (May-August) prior to the first rainfall of the wet season. The loss of physiological dormancy enabled initiation of embryo growth within the seed during the first 2 months of the rainy season (September-October), resulting in a germination peak in November. Embryo growth occurred mainly through cell expansion but some dividing cells were also observed. Endosperm digestion started at the micropylar side around the embryo and diffused to the rest of the endosperm. Exogenous gibberellins induced both embryo growth and endo-beta-mannanase activity in dormant seeds.
Conclusions: The physiological dormancy component is broken by low temperature and/or temperature fluctuations preceding the rainy season. Subsequent embryo growth and digestion of the endosperm are both likely to be controlled by gibberellins synthesized during the breaking of physiological dormancy. Radicle protrusion thus occurred at the beginning of the rainy season, thereby maximizing the opportunity for seedlings to emerge and establish.
Figures






References
-
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998.
-
- Buckeridge MS, Tiné MAS, Santos HP, Lima DU. Polissacarídeos de reserva de parede celular em sementes. Estrutura, metabolismo, funções e aspectos ecológicos. Revista Brasileira de Fisiologia Vegetal. 2000;12:137–162.
-
- Bouwmeester HJ, Karssen CM. Annual changes in dormancy and germination in seeds of Sisymbrium officinale (L.) Scop. New Phytologist. 1993;124:179–191.
-
- Correia MP. In: Dicionário de plantas úteis e espécies exóticas. de Janeiro Rio., editor. Imprensa Nacional, Ministério da Agricultura; 1926.
-
- Derkx MPM, Vermeer E, Karssen CM. Gibberellins in seeds of Arabidopsis thaliana: biological activities, identifications and effect of light and chilling on endogenous levels. Plant Growth Regulation. 1994;15:223–234.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

