Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress
- PMID: 17329412
- PMCID: PMC6673487
- DOI: 10.1523/JNEUROSCI.4945-06.2007
Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress
Abstract
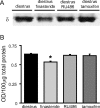
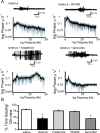
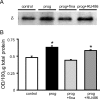
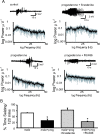
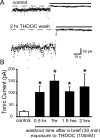
Recently, we demonstrated cyclic alterations in GABA(A) receptor (GABA(A)R) subunit composition over the ovarian cycle correlated with fluctuations in progesterone levels. However, it remains unclear whether this physiological regulation of GABA(A)Rs is directly mediated by hormones. Here, we show that both ovarian and stress hormones are capable of reorganizing GABA(A)Rs by actions through neurosteroid metabolites. The cyclic alterations in GABA(A)Rs demonstrated in female mice can be mimicked with exogenous progesterone treatment in males or in ovariectomized females. Progesterone (5 mg/kg, twice daily) upregulates the expression of GABA(A)R delta subunits and enhances the tonic inhibition mediated by these receptors in dentate gyrus granule cells (DGGCs). These changes in males as well as ovarian cycle-induced changes in females can be blocked by finasteride, an antagonist of neurosteroid synthesis from progesterone. The altered GABA(A)R expression is unaffected by the progesterone receptor antagonist RU486 [mifepristone (11beta-[p-(dimethylamino)phenyl]-17beta-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one)], suggesting that neurosteroid synthesis and not progesterone receptor activation underlies the hormone-mediated effects on GABA(A)R expression. Neurosteroids can alter GABA(A)R expression on a rapid timescale, because GABA(A)R upregulation can be induced in brain slices maintained in vitro after a short (30 min) treatment with the neurosteroid 3alpha,5alpha-tetrahydrodeoxycorticosterone (THDOC) (100 nM). Consistent with these rapid alterations, acute stress, a condition known to quickly raise THDOC levels, within 30 min induces upregulation of GABA(A)R delta subunit expression and increase tonic inhibition in DGGCs. These results reveal that several physiological conditions characterized by elevations in neurosteroid levels induce a reorganization of GABA(A)Rs through the action of neurosteroids.
Figures


References
-
- Akinci MK, Johnston GA. Sex differences in acute swim stress-induced changes in the binding of MK-801 to the NMDA subclass of glutamate receptors in mouse forebrain. J Neurochem. 1993;61:2290–2293. - PubMed
-
- Barbaccia ML, Roscetti G, Trabucchi M, Cuccheddu T, Concas A, Biggio G. Neurosteroids in the brain of handling-habituated and naive rats: effect of CO2 inhalation. Eur J Pharmacol. 1994;261:317–320. - PubMed
-
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABA(A) receptor function after acute stress. Neuroendocrinology. 1996a;63:166–172. - PubMed
-
- Barbaccia ML, Concas A, Roscetti G, Bolacchi F, Mostallino MC, Purdy RH, Biggio G. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996b;54:205–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
