Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans
- PMID: 17329414
- PMCID: PMC2848955
- DOI: 10.1523/JNEUROSCI.5176-06.2007
Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans
Abstract
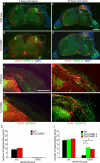
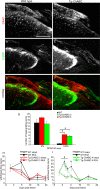
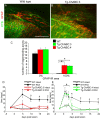
Axotomized neurons within the damaged CNS are thought to be prevented from functional regeneration by inhibitory molecules such as chondroitin sulfate proteoglycans (CSPGs) and myelin-associated inhibitors. Here, we provide a transgenic test of the role of CSPGs in limiting regeneration, using the gfap promotor to express a CSPG-degrading enzyme chondroitinase ABC (ChABC) in astrocytes. Corticospinal axons extend within the lesion site, but not caudal to it, after dorsal hemisection in the transgenic mice. The presence of the gfap-ChABC transgene yields no significant improvement in motor function recovery in this model. In contrast, functionally significant sensory axon regeneration is observed after dorsal rhizotomy in transgenic mice. These transgenic studies confirm a local efficacy for reduced CSPG to enhance CNS axon growth after traumatic injury. CSPGs appear to function in a spatially distinct role from myelin inhibitors, implying that combination-based therapy will be especially advantageous for CNS injuries.
Figures
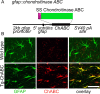
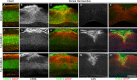
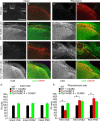
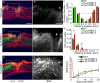
References
-
- Abad F, Feria M, Boada J. Chronic amitriptyline decreases autotomy following dorsal rhizotomy in rats. Neurosci Lett. 1989;99:187–190. - PubMed
-
- Aldskogius H, Kozlova EN. Central neuron-glial and glial-glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
