Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation
- PMID: 17329423
- PMCID: PMC6673482
- DOI: 10.1523/JNEUROSCI.5582-06.2007
Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation
Abstract
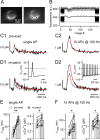
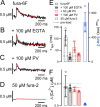
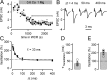
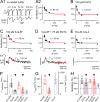
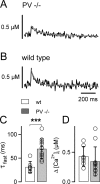
Presynaptic Ca2+ signaling plays a crucial role in short-term plasticity of synaptic transmission. Here, we studied the role of mobile endogenous presynaptic Ca2+ buffer(s) in modulating paired-pulse facilitation at a large excitatory nerve terminal in the auditory brainstem, the calyx of Held. To do so, we assessed the effect of presynaptic whole-cell recording, which should lead to the diffusional loss of endogenous mobile Ca2+ buffers, on paired-pulse facilitation and on intracellular Ca2+ concentration ([Ca2+]i) transients evoked by action potentials. In unperturbed calyces briefly preloaded with the Ca2+ indicator fura-6F, the [Ca2+]i transient decayed surprisingly fast (tau(fast), approximately 30 ms). Presynaptic whole-cell recordings made without additional Ca2+ buffers slowed the decay kinetics of [Ca2+]i and paired-pulse facilitation (twofold to threefold), but the amplitude of the [Ca2+]i transient was changed only marginally. The fast [Ca2+]i decay was restored by adding the slow Ca2+ buffer EGTA (50-100 microM) or parvalbumin (100 microM), a Ca2+-binding protein with slow Ca2+-binding kinetics, to the presynaptic pipette solution. In contrast, the fast Ca2+ buffer fura-2 strongly reduced the amplitude of the [Ca2+]i transient and slowed its decay, suggesting that the mobile endogenous buffer in calyces of Held has slow, rather than fast, binding kinetics. In parvalbumin knock-out mice, the decay of [Ca2+]i and facilitation was slowed approximately twofold compared with wild-type mice, similar to what is observed during whole-cell recordings in rat calyces of Held. Thus, in young calyces of Held, a mobile Ca2+ buffer with slow binding kinetics, primarily represented by parvalbumin, accelerates the decay of spatially averaged [Ca2+]i and paired-pulse facilitation.
Figures


Similar articles
-
Pre- and postsynaptic whole-cell recordings in the medial nucleus of the trapezoid body of the rat.J Physiol. 1995 Dec 15;489 ( Pt 3)(Pt 3):825-40. doi: 10.1113/jphysiol.1995.sp021095. J Physiol. 1995. PMID: 8788946 Free PMC article.
-
Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling.J Neurosci. 2005 Jan 5;25(1):96-107. doi: 10.1523/JNEUROSCI.3748-04.2005. J Neurosci. 2005. PMID: 15634771 Free PMC article.
-
Developmental expression of the Ca2+-binding proteins calretinin and parvalbumin at the calyx of Held of rats and mice.Eur J Neurosci. 2004 Sep;20(6):1473-82. doi: 10.1111/j.1460-9568.2004.03604.x. Eur J Neurosci. 2004. PMID: 15355314
-
Transients in global Ca2+ concentration induced by electrical activity in a giant nerve terminal.J Physiol. 2013 Jul 1;591(13):3189-95. doi: 10.1113/jphysiol.2012.248617. Epub 2013 Mar 25. J Physiol. 2013. PMID: 23529127 Free PMC article. Review.
-
Multiple overlapping processes underlying short-term synaptic enhancement.Trends Neurosci. 1997 Apr;20(4):170-7. doi: 10.1016/s0166-2236(96)01001-6. Trends Neurosci. 1997. PMID: 9106358 Review.
Cited by
-
The Parvalbumin Hypothesis of Autism Spectrum Disorder.Front Cell Neurosci. 2020 Dec 18;14:577525. doi: 10.3389/fncel.2020.577525. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33390904 Free PMC article.
-
Synaptotagmin-2 is a reliable marker for parvalbumin positive inhibitory boutons in the mouse visual cortex.PLoS One. 2012;7(4):e35323. doi: 10.1371/journal.pone.0035323. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22539967 Free PMC article.
-
Prolactin Reduces Hippocampal Parvalbumin and GABAA Receptor Expression in Female Mice.Neuroendocrinology. 2022;112(8):796-806. doi: 10.1159/000520279. Epub 2021 Oct 19. Neuroendocrinology. 2022. PMID: 34666336 Free PMC article.
-
Identification of mouse soleus muscle proteins altered in response to changes in gravity loading.Sci Rep. 2023 Sep 22;13(1):15768. doi: 10.1038/s41598-023-42875-8. Sci Rep. 2023. PMID: 37737267 Free PMC article.
-
Dynamics of volume-averaged intracellular Ca2+ in a rat CNS nerve terminal during single and repetitive voltage-clamp depolarizations.J Physiol. 2017 May 15;595(10):3219-3236. doi: 10.1113/JP272773. Epub 2017 Feb 1. J Physiol. 2017. PMID: 27957749 Free PMC article.
References
-
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. - PubMed
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
