Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study
- PMID: 17329424
- PMCID: PMC6673473
- DOI: 10.1523/JNEUROSCI.3470-06.2007
Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study
Abstract
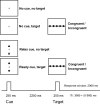
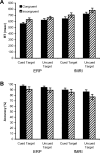
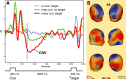
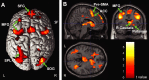
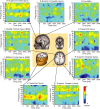
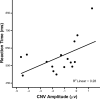
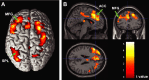
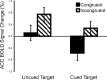
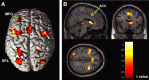
Response anticipation and response conflict processes are supported by executive control. However, few neuroimaging studies have attempted to study the relationship between these two processes in the same experimental session. In this study, we isolated brain activity associated with response anticipation (after a cue to prepare vs relax) and with response conflict (responding to a target with incongruent vs congruent flankers) and examined the independence and interaction of brain networks supporting these processes using event-related potentials (ERPs) and functional magnetic resonance imaging. Response anticipation generated a contingent negative variation ERP that correlated with shorter reaction times, and was associated with activation of a thalamo-cortico-striatal network, as well as increased gamma band power in frontal and parietal regions, and decreased spectral power in theta, alpha, and beta bands in most regions. Response conflict was associated with increased activation in the anterior cingulate cortex (ACC) and prefrontal cortex of the executive control network, with an overlap in activation with response anticipation in regions including the middle frontal gyrus, ACC, and superior parietal lobule. Although the executive control network showed increased activation in response to unanticipated versus anticipated targets, the response conflict effect was not altered by response anticipation. These results suggest that common regions of a dorsal frontoparietal network and the ACC are engaged in the flexible control of a wide range of executive processes, and that response anticipation modulates overall activity in the executive control network but does not interact with response conflict processing.
Figures
References
-
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. - PubMed
-
- Andrew C, Pfurtscheller G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol. 1996;98:144–148. - PubMed
-
- Barcelo F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. - PubMed
-
- Bastiaansen MC, Brunia CH. Anticipatory attention: an event-related desynchronization approach. Int J Psychophysiol. 2001;43:91–107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
