Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes
- PMID: 17329428
- PMCID: PMC6673489
- DOI: 10.1523/JNEUROSCI.4565-06.2007
Development of hydrocephalus in mice expressing the G(i)-coupled GPCR Ro1 RASSL receptor in astrocytes
Abstract
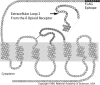
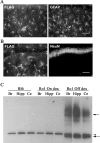
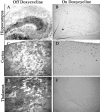
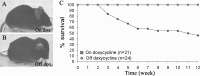
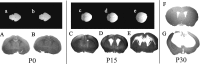
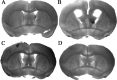
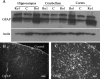
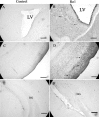
We developed a transgenic mouse line that expresses the G(i)-coupled RASSL (receptor activated solely by synthetic ligand) Ro1 in astrocytes to study astrocyte-neuronal communication. Surprisingly, we found that all transgenics expressing Ro1 developed hydrocephalus. We analyzed these mice in an effort to develop a new model of hydrocephalus that will further our understanding of the pathophysiology of the disease. Expression of Ro1 was restricted to astrocytes by crossing the transgenic hGFAP-tTA (tet transactivator behind the human glial fibrillary acidic protein promoter) mouse line with the transgenic tetO-Ro1/tetO-LacZ mouse line. This cross produced double-transgenic mice that expressed Ro1 in astrocytes. All double transgenics developed hydrocephalus by postnatal day 15, whereas single-transgenic littermate controls appeared normal. Hydrocephalic Ro1 mice displayed enlarged ventricles, partial denudation of the ependymal cell layer, altered subcommissural organ morphology, and obliteration of the cerebral aqueduct. Severely hydrocephalic mice also had increased levels of phospho-Erk and GFAP expression. Administration of doxycycline to breeding pairs suppressed Ro1 expression and the onset of hydrocephalus in double-transgenic offspring. Ro1 animals maintained on dox did not develop hydrocephalus; however, if taken off doxycycline at weaning, double-transgenic mice developed enlarged ventricles within 7 weeks, indicating that Ro1 expression also induces hydrocephalus in adults. This study discovered a new model of hydrocephalus in which the rate of pathogenesis can be controlled enabling the study of the pathogenesis of both juvenile and adult onset hydrocephalus.
Figures

References
-
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. - PubMed
-
- Bach A, Lallemand Y, Nicola MA, Ramos C, Mathis L, Maufras M, Robert B. Msx1 is required for dorsal diencephalon patterning. Development. 2003;130:4025–4036. - PubMed
-
- Blackshear PJ, Graves JP, Stumpo DJ, Cobos I, Rubenstein JL, Zeldin DC. Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development. 2003;130:4539–4552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
