Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations
- PMID: 17329429
- PMCID: PMC6673471
- DOI: 10.1523/JNEUROSCI.4322-06.2007
Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations
Abstract
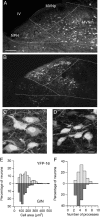
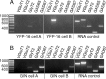
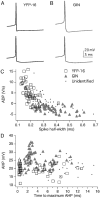
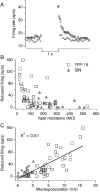
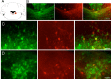
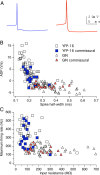
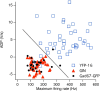
The identification of neuron types within circuits is fundamental to understanding their relevance to behavior. In the vestibular nuclei, several classes of neurons have been defined in vivo on the basis of their activity during behavior, but it is unclear how those types correspond to neurons identified in slice preparations. By targeting recordings to neurons labeled in transgenic mouse lines, this study reveals that the continuous distribution of intrinsic parameters observed in medial vestibular nucleus (MVN) neurons can be neatly subdivided into two populations of neurons, one of which is GABAergic and the other of which is exclusively glycinergic or glutamatergic. In slice recordings, GABAergic neurons labeled in the EGFP (enhanced green fluorescent protein)-expressing inhibitory neuron (GIN) line displayed lower maximum firing rates (<250 Hz) than glycinergic and glutamatergic neurons labeled in the yellow fluorescent protein-16 (YFP-16) line (up to 500 Hz). In contrast to cortical and hippocampal interneurons, GABAergic MVN neurons exhibited wider action potentials than glutamatergic (and glycinergic) neurons. Responses to current injection differed between the neurons labeled in the two lines, with GIN neurons modulating their firing rates over a smaller input range, adapting less during steady depolarization, and exhibiting less rebound firing than YFP-16 neurons. These results provide a scheme for robust classification of unidentified MVN neurons by their physiological properties. Finally, dye labeling in slices shows that both GABAergic and glycinergic neurons project to the contralateral vestibular nuclei, indicating that commissural inhibition is accomplished through at least two processing streams with differential input and output properties.
Figures

References
-
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34:1011–1020. - PubMed
-
- Barmack NH, Fagerson M, Errico P. Cholinergic projection to the dorsal cap of the inferior olive of the rat, rabbit, and monkey. J Comp Neurol. 1993;328:263–281. - PubMed
-
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. 2003;90:184–203. - PubMed
-
- Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. 2004;92:1668–1684. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
