Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes
- PMID: 17329434
- PMCID: PMC6673492
- DOI: 10.1523/JNEUROSCI.3565-06.2007
Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes
Abstract
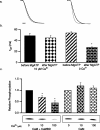
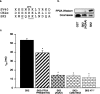
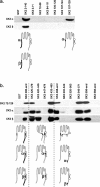
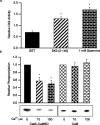
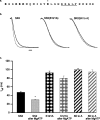
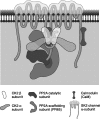
Small conductance Ca2+-activated K+ channels (SK channels) are complexes of four alpha pore-forming subunits each bound by calmodulin (CaM) that mediate Ca2+ gating. Proteomic analysis indicated that SK2 channels also bind protein kinase CK2 (CK2) and protein phosphatase 2A (PP2A). Coexpression of SK2 with the CaM phosphorylation surrogate CaM(T80D) suggested that the apparent Ca2+ sensitivity of SK2 channels is reduced by CK2 phosphorylation of SK2-bound CaM. By using 4,5,6,7-tetrabromo-2-azabenzimidazole, a CK2-specific inhibitor, we confirmed that SK2 channels coassemble with CK2. PP2A also binds to SK2 channels and counterbalances the effects of CK2, as shown by coexpression of a dominant-negative mutant PP2A as well as a mutant SK2 channel no longer able to bind PP2A. In vitro binding studies have revealed interactions between the N and C termini of the channel subunits as well as interactions among CK2 alpha and beta subunits, PP2A, and distinct domains of the channel. In the channel complex, lysine residue 121 within the N-terminal domain of the channel activates SK2-bound CK2, and phosphorylation of CaM is state dependent, occurring only when the channels are closed. The effects of CK2 and PP2A indicate that native SK2 channels are multiprotein complexes that contain constitutively associated CaM, both subunits of CK2, and at least two different subunits of PP2A. The results also show that the Ca2+ sensitivity of SK2 channels is regulated in a dynamic manner, directly through CK2 and PP2A, and indirectly by Ca2+ itself via the state dependence of CaM phosphorylation by CK2.
Figures

References
-
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–335. - PubMed
-
- Arrigoni G, Marin O, Pagano MA, Settimo L, Paolin B, Meggio F, Pinna LA. Phosphorylation of calmodulin fragments by protein kinase CK2: mechanistic aspects and structural consequences. Biochemistry. 2004;43:12788–12798. - PubMed
-
- Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. - PubMed
-
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
