Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines
- PMID: 17329444
- PMCID: PMC1865614
- DOI: 10.1128/CVI.00387-06
Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines
Abstract
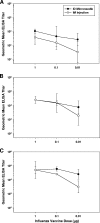
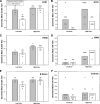
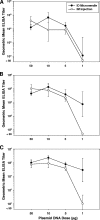
Recent clinical studies have suggested that, for certain strains of influenza virus, intradermal (i.d.) delivery may enable protective immune responses using a lower dose of vaccine than required by intramuscular (i.m.) injection. Here, we describe the first preclinical use of microneedle technology for i.d. administration of three different types of influenza vaccines: (i) a whole inactivated influenza virus, (ii) a trivalent split-virion human vaccine, and (iii) a plasmid DNA encoding the influenza virus hemagglutinin. In a rat model, i.d. delivery of the whole inactivated virus provided up to 100-fold dose sparing compared to i.m. injection. In addition, i.d. delivery of the trivalent human vaccine enabled at least 10-fold dose sparing for the H1N1 strain and elicited levels of response across the dose range similar to those of i.m. injection for the H3N2 and B strains. Furthermore, at least fivefold dose sparing from i.d. delivery was evident in animals treated with multiple doses of DNA plasmid vaccine, although such effects were not apparent after the first immunization. Altogether, the results demonstrate that microneedle-based i.d. delivery elicits antibody responses that are at least as strong as via i.m. injection and that, in many cases, dose sparing can be achieved by this new immunization method.
Figures


References
-
- Alchas, P. G. December 2002. Intradermal delivery device including a needle assembly. U.S. patent 6,494,865.
-
- Alchas, P. G., C. E. Guillermo, and M. S. Korisch. May 2003. Prefillable intradermal injector. U.S. patent 6,569,123.
-
- Alchas, P. G., and P. Laurent. May 2003. Method of intradermally injecting substances. U.S. patent 6,569,143.
-
- Alchas, P. G., P. Laurent, C. E. Guillermo, and M. S. Korisch. January 2005. Intradermal needle. U.S. patent 6,843,781.
-
- Audsley, J. M., and G. A. Tannock. 2004. The role of cell culture vaccines in the control of the next influenza pandemic. Exp. Opin. Biol. Ther. 4:709-717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

