Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design
- PMID: 17329445
- PMCID: PMC1865631
- DOI: 10.1128/CVI.00371-06
Antibody responses are generated to immunodominant ELK/KLE-type motifs on the nonstructural-1 glycoprotein during live dengue virus infections in mice and humans: implications for diagnosis, pathogenesis, and vaccine design
Abstract
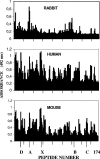
Antibodies generated to the purified dengue type 2 virus (D-2V) nonstructural-1 (NS1) protein in mice and rabbits were compared with those generated to this protein in congeneic (H-2 class II) mouse strains and humans after D-2V infections. Unlike the profiles observed with the rabbits, similar antibody reaction profiles were generated by mice and humans with severe D-2V disease (dengue hemorrhagic fever [DHF]/dengue shock syndrome [DSS]). Many of these epitopes contained the core acidic-hydrophobic-basic (tri-amino-acid; ELK-type) motifs present in the positive or negative orientations. Antibody responses generated to these ELK/KLE-type motifs and the epitope LX1 on this protein were influenced by class II molecules in mice during D-2V infections; but these antibodies cross-reacted with human fibrinogen and platelets, as implicated in DHF/DSS pathogenesis. The core LX1 epitope (113YSWKTWG119), identified by the dengue virus complex-specific monoclonal antibody (MAb) 3D1.4, was prepared so that it contained natural I-Ad-binding and ELK-type motifs. This AFLX1 peptide, which appropriately displayed the ELK-type and LX1 epitopes in solid-phase immunoassays, generated a similar, but lower, immunodominant anti-ELK-motif antibody reaction in I-Ad-positive mice, as generated in mice and humans during D-2V infections. These antibody responses were much stronger in the high-responding mouse strains and each of the DHF/DSS patients tested and may therefore account for the association of DHF/DSS resistance or susceptibility with particular class II molecules and autoantibodies, antibody-stimulating cytokines (e.g., interleukin-6), and complement product C3a being implicated in DHF/DSS pathogenesis. These results are likely to be important for the design of a safe vaccine against this viral disease and showed the AFLX1 peptide and MAb 3D1.4 to be valuable diagnostic reagents.
Figures
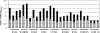
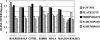


Similar articles
-
Antibody responses to an immunodominant nonstructural 1 synthetic peptide in patients with dengue fever and dengue hemorrhagic fever.J Med Virol. 1999 Jan;57(1):1-8. J Med Virol. 1999. PMID: 9890415
-
The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis.Arch Virol. 1997;142(5):897-916. doi: 10.1007/s007050050127. Arch Virol. 1997. PMID: 9191856
-
Monoclonal antibodies that bind to common epitopes on the dengue virus type 2 nonstructural-1 and envelope glycoproteins display weak neutralizing activity and differentiated responses to virulent strains: implications for pathogenesis and vaccines.Clin Vaccine Immunol. 2008 Mar;15(3):549-61. doi: 10.1128/CVI.00351-07. Epub 2007 Dec 26. Clin Vaccine Immunol. 2008. PMID: 18160621 Free PMC article.
-
Autoimmune pathogenesis in dengue virus infection.Viral Immunol. 2006 Summer;19(2):127-32. doi: 10.1089/vim.2006.19.127. Viral Immunol. 2006. PMID: 16817755 Review.
-
Alternate hypothesis on the pathogenesis of dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) in dengue virus infection.Exp Biol Med (Maywood). 2008 Apr;233(4):401-8. doi: 10.3181/0707-MR-198. Exp Biol Med (Maywood). 2008. PMID: 18367628 Review.
Cited by
-
Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines.PLoS One. 2011;6(10):e25685. doi: 10.1371/journal.pone.0025685. Epub 2011 Oct 21. PLoS One. 2011. PMID: 22031819 Free PMC article.
-
Linear and Continuous Flavivirus Epitopes From Naturally Infected Humans.Front Cell Infect Microbiol. 2021 Aug 12;11:710551. doi: 10.3389/fcimb.2021.710551. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34458161 Free PMC article. Review.
-
Kinetics of NS1 and anti-NS1 IgG following dengue infection reveals likely early formation of immune complexes in secondary infected patients.Sci Rep. 2025 Feb 25;15(1):6684. doi: 10.1038/s41598-025-91099-5. Sci Rep. 2025. PMID: 39994315 Free PMC article.
-
Metadata-driven comparative analysis tool for sequences (meta-CATS): an automated process for identifying significant sequence variations that correlate with virus attributes.Virology. 2013 Dec;447(1-2):45-51. doi: 10.1016/j.virol.2013.08.021. Epub 2013 Sep 14. Virology. 2013. PMID: 24210098 Free PMC article.
-
Antibodies Against Modified NS1 Wing Domain Peptide Protect Against Dengue Virus Infection.Sci Rep. 2017 Aug 1;7(1):6975. doi: 10.1038/s41598-017-07308-3. Sci Rep. 2017. PMID: 28765561 Free PMC article.
References
-
- Alcon, S., A Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. - PMC - PubMed
-
- Bhamarapravati, N. 1997. Pathology of dengue infections, p. 115-132. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, New York, NY.
-
- Churdboonchart, V., N. Bhamarapravati, S. Peampramprecha, and S. Sirinavin. 1991. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 44:481-493. - PubMed
-
- Creech, E. A., D. Nakul-Aguaronne, E. A. Reap, R. L. Cheek, P. A. Wolthusen, P. L. Cohen, and R. A. Eisenburg. 1996. MHC genes modify systemic autoimmune disease. The role of the I-E locus. J. Immunol. 156:812-817. - PubMed
-
- Falconar, A. K. I. 1999. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-reactive monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol. 144:2313-2330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

