Crystallization, diffraction data collection and preliminary crystallographic analysis of hexagonal crystals of Pseudomonas aeruginosa amidase
- PMID: 17329817
- PMCID: PMC2330192
- DOI: 10.1107/S1744309107005830
Crystallization, diffraction data collection and preliminary crystallographic analysis of hexagonal crystals of Pseudomonas aeruginosa amidase
Abstract
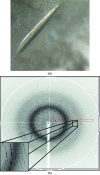
The aliphatic amidase (acylamide amidohydrolase; EC 3.5.1.4) from Pseudomonas aeruginosa is a hexameric enzyme composed of six identical subunits with a molecular weight of approximately 38 kDa. Since microbial amidases are very important enzymes in industrial biocatalysis, the structural characterization of this enzyme will help in the design of novel catalytic activities of commercial interest. The present study reports the successful crystallization of the wild-type amidase from P. aeruginosa. Native crystals were obtained and a complete data set was collected at 1.4 A resolution, although the crystals showed diffraction to 1.25 A resolution. The crystals were found to belong to space group P6(3)22, with unit-cell parameters a = b = 102.60, c = 151.71 A, and contain one molecule in the asymmetric unit.
Figures
Similar articles
-
Evidence that cysteine-166 is the active-site nucleophile of Pseudomonas aeruginosa amidase: crystallization and preliminary X-ray diffraction analysis of the enzyme.Biochem J. 1999 Jun 15;340 ( Pt 3)(Pt 3):711-4. Biochem J. 1999. PMID: 10359655 Free PMC article.
-
Crystallization of and preliminary X-ray data for the negative regulator (AmiC) of the amidase operon of Pseudomonas aeruginosa.J Mol Biol. 1991 Dec 20;222(4):869-71. doi: 10.1016/0022-2836(91)90579-u. J Mol Biol. 1991. PMID: 1762155
-
Crystallization and preliminary X-ray crystallographic analysis of peptide deformylase from Pseudomonas aeruginosa.Acta Crystallogr D Biol Crystallogr. 2002 Oct;58(Pt 10 Pt 2):1874-5. doi: 10.1107/s0907444902013835. Epub 2002 Sep 28. Acta Crystallogr D Biol Crystallogr. 2002. PMID: 12351843
-
New results using Laue diffraction and time-resolved crystallography.Curr Opin Struct Biol. 1998 Oct;8(5):612-8. doi: 10.1016/s0959-440x(98)80153-x. Curr Opin Struct Biol. 1998. PMID: 9818266 Review.
-
The growth and preliminary investigation of protein and nucleic acid crystals for X-ray diffraction analysis.Methods Biochem Anal. 1976;23(0):249-345. doi: 10.1002/9780470110430.ch4. Methods Biochem Anal. 1976. PMID: 12447 Review. No abstract available.
Cited by
-
Acetamide hydrolyzing activity of Bacillus megaterium F-8 with bioremediation potential: optimization of production and reaction conditions.Environ Sci Pollut Res Int. 2014;21(14):8822-30. doi: 10.1007/s11356-014-2818-7. Epub 2014 Apr 11. Environ Sci Pollut Res Int. 2014. PMID: 24723348
References
-
- Banerjee, A., Sharma, R. & Banerjee, U. C. (2002). Appl. Microbiol. Biotechnol.60, 33–44. - PubMed
-
- Brown, J. E., Brown, P. R. & Clarke, P. H. (1969). J. Gen. Microbiol.57, 273–285. - PubMed
-
- Brown, P. R. & Clarke, P. H. (1972). J. Gen. Microbiol.70, 287–288. - PubMed
-
- Brown, P. R. & Tata, R. (1987). J. Gen. Microbiol.133, 1527–1533. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

