Autophagy, mitochondria and cell death in lysosomal storage diseases
- PMID: 17329960
- PMCID: PMC2777544
- DOI: 10.4161/auto.3906
Autophagy, mitochondria and cell death in lysosomal storage diseases
Abstract
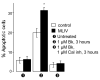
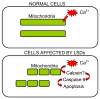
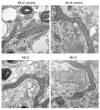
Lysosomal storage diseases (LSDs) are debilitating genetic conditions that frequently manifest as neurodegenerative disorders. They severely affect eye, motor and cognitive functions and, in most cases, abbreviate the lifespan. Postmitotic cells such as neurons and mononuclear phagocytes rich in lysosomes are most often affected by the accumulation of undegraded material. Cell death is well documented in parts of the brain and in other cells of LSD patients and animal models, although little is known about mechanisms by which death pathways are activated in these diseases, and not all cells exhibiting increased storage material are affected by cell death. Lysosomes are essential for maturation and completion of autophagy-initiated protein and organelle degradation. Moreover, accumulation of effete mitochondria has been documented in postmitotic cells whose lysosomal function is suppressed or in aging cells with lipofuscin accumulation. Based upon observations in the literature and our own data showing similar mitochondrial abnormalities in several LSDs, we propose a new model of cell death in LSDs. We suggest that the lysosomal deficiencies in LSDs inhibit autophagic maturation, leading to a condition of autophagic stress. The resulting accumulation of dysfunctional mitochondria showing impaired Ca2+ buffering increases the vulnerability of the cells to pro-apoptotic signals.
Figures

References
-
- Mancini GM, Havelaar AC, Verheijen FW. Lysosomal transport disorders. J Inherit Metab Dis. 2000;23:278–92. - PubMed
-
- Mach L. Biosynthesis of lysosomal proteinases in health and disease. Biol Chem. 2002;383:751–6. - PubMed
-
- Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. - PubMed
-
- Sleat DE, Wiseman JA, El-Banna M, Kim KH, Mao Q, Price S, Macauley SL, Sidman RL, Shen MM, Zhao Q, Passini MA, Davidson BL, Stewart GR, Lobel P. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J Neurosci. 2004;24:9117–26. - PMC - PubMed
-
- Duggleby W, Bateman J, Singer S. The aging experience of well elderly women: initial results. Nurs Health Sci. 2002;4:A10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
