Alteration of the cell adhesion molecule L1 expression in a specific subset of primary afferent neurons contributes to neuropathic pain
- PMID: 17331206
- PMCID: PMC1891330
- DOI: 10.1111/j.1460-9568.2007.05344.x
Alteration of the cell adhesion molecule L1 expression in a specific subset of primary afferent neurons contributes to neuropathic pain
Abstract
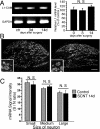
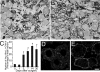
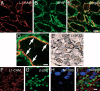
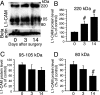
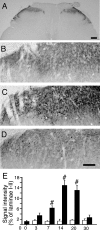
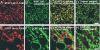
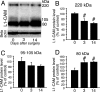
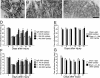
The cell adhesion molecule L1 (L1-CAM) plays important functional roles in the developing and adult nervous systems. Here we show that peripheral nerve injury induced dynamic post-transcriptional alteration of L1-CAM in the rat dorsal root ganglia (DRGs) and spinal cord. Sciatic nerve transection (SCNT) changed the expression of L1-CAM protein but not L1-CAM mRNA. In DRGs, SCNT induced accumulation of the L1-CAM into the surface of somata, which resulted in the formation of immunoreactive ring structures in a number of unmyelinated C-fiber neurons. These neurons with L1-CAM-immunoreactive ring structures were heavily colocalized with phosphorylated p38 MAPK. Western blot analysis revealed the increase of full-length L1-CAM and decrease of fragments of L1-CAM after SCNT in DRGs. Following SCNT, L1-CAM-immunoreactive profiles in the dorsal horn showed an increase mainly in pre-synaptic areas of laminae I-II with a delayed onset and colocalized with growth-associated protein 43. In contrast to DRGs, SCNT increased the proteolytic 80-kDa fragment of L1-CAM and decreased full-length L1-CAM in the spinal cord. The intrathecal injection of L1-CAM antibody for the extracellular domain of L1-CAM inhibited activation of p38 MAPK and emergence of ring structures of L1-CAM immunoreactivity in injured DRG neurons. Moreover, inhibition of extracellular L1-CAM binding by intrathecal administration of antibody suppressed the mechanical allodynia and thermal hyperalgesia induced by partial SCNT. Collectively, these data suggest that the modification of L1-CAM in nociceptive pathways might be an important pathomechanism of neuropathic pain.
Figures




References
-
- Aoki E. Localization of nitric oxide-related substances in the peripheral nervous tissues. Brain Res. 1993;620:142–145. - PubMed
-
- Baba H. Silent NMDA receptor-mediated synapses are developmentally regulated in the dorsal horn of the rat spinal cord. JNeurophysiol. 2000;83:955–962. - PubMed
-
- Benowitz LI. GAP-43 as a marker for structural plasticity in the mature CNS. ProgBrain Res. 1990;86:309–320. - PubMed
-
- Brummendorf T. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

