The funding and use of high-cost medicines in Australia: the example of anti-rheumatic biological medicines
- PMID: 17331230
- PMCID: PMC1828161
- DOI: 10.1186/1743-8462-4-2
The funding and use of high-cost medicines in Australia: the example of anti-rheumatic biological medicines
Abstract
Background: Subsidised access to high-cost medicines in Australia is restricted under national programs (the Pharmaceutical Benefits Scheme, PBS, and the Repatriation Pharmaceutical Benefits Scheme, RPBS) with a view to achieving cost-effective use. The aim of this study was to examine the use and associated government cost of biological agents for treating rheumatoid arthritis over the first two years of subsidy, and to compare these data to the predicted outcomes.
Methods: National prescription and expenditure data for the biologicals, etanercept, infliximab, adalimumab, and anakinra were collected and analysed for the period August 2003 to July 2005. Dispensing data on biologicals sorted by the metropolitan, rural and remote zones and by prescriber major specialty were also examined.
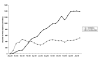
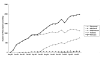
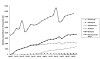
Results: A total of 27,970 prescriptions for biologicals was reimbursed. The government expenditure was A$53.1 million, representing only 19% of that expected. Almost all prescriptions were reimbursed by the PBS (98%, A$52 million) and the remainder by the RPBS. Approximately 62% of the prescriptions were for concessional patients (A$32.9 million). There was considerable variability in the use of biologicals across Australian states and territories, usage roughly correlating with the per capita adjusted number of rheumatologists. The total number of prescriptions continued to increase over the study period. Etanercept was the most highly prescribed agent (74% by number of prescriptions), although its use was beginning to plateau. Use of adalimumab increased steadily. Use of infliximab and anakinra was considerably lower. The resultant health outcomes for individual patients are unknown. Prescribers from capital cities and other metropolitan centres provided a majority of prescriptions of biologicals (89%).
Conclusion: The overall uptake of biologicals for treating rheumatoid arthritis over the first two years of PBS subsidy was considerably lower than expected. Long-term safety concerns and the expanded clinical uses of these drugs emphasise the need for evaluation. It is essential that there is comprehensive, ongoing analysis of utilisation data, associated expenditure and, importantly, patient outcomes in order to enhance accountability, efficiency and equity of policies that allocate substantial resources to subsidising national access to high-cost medicines.
Figures
References
-
- Organisation for Economic Co-operation and Development (OECD) OECD Health Data: Statistics and indicator for 30 countries. 2005.
-
- Australian Government Productivity Commission . International pharmaceutical price differences: research report. Canberra, Australian Government, Productivity Commission; 2001.
-
- Australian Institute of Health and Welfare Health expenditure Australia 2003-04. AIHW Cat. No. HWE 32. Canberra: AIHW. 2005.
-
- Commonwealth Department of Health and Aged Care . The Australian Health Care System, An Outline. Canberra, ACT, Publications Unit, Commonwealth Department of Health and Aged Care; 2000.
-
- Henry D. Economic analysis as an aid to subsidisation decisions: the development of Australian guidelines for pharmaceuticals. pharmacoeconomics. 1992;1:54–67. - PubMed
LinkOut - more resources
Full Text Sources

