Caveolin-1 sensitizes rat pituitary adenoma GH3 cells to bromocriptine induced apoptosis
- PMID: 17331262
- PMCID: PMC1832175
- DOI: 10.1186/1475-2867-7-1
Caveolin-1 sensitizes rat pituitary adenoma GH3 cells to bromocriptine induced apoptosis
Abstract
Background: Prolactinoma is the most frequent pituitary tumor in humans. The dopamine D2 receptor agonist bromocriptine has been widely used clinically to treat human breast tumor and prolactinoma through inhibition of hyperprolactinemia and induction of tumor cell apoptosis, respectively, but the molecular mechanism of bromocriptine induction of pituitary tumor apoptosis remains unclear. Caveolin-1 is a membrane-anchored protein enriched on caveolae, inverted flask-shaped invaginations on plasma membranes where signal transduction molecules are concentrated. Currently, caveolin-1 is thought to be a negative regulator of cellular proliferation and an enhancer of apoptosis by blocking signal transduction between cell surface membrane receptors and intracellular signaling protein cascades. Rat pituitary adenoma GH3 cells, which express endogenous caveolin-1, exhibit increased apoptosis and shrinkage after exposure to bromocriptine. Hence, the GH3 cell line is an ideal model for studying the molecular action of bromocriptine on prolactinoma.
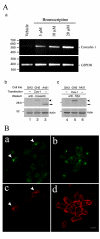
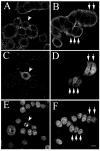
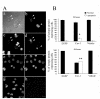
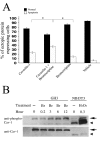
Results: The expression of endogenous caveolin-1 in GH3 cells was elevated after bromocriptine treatment. Transiently expressed mouse recombinant caveolin-1 induced apoptosis in GH3 cells by enhancing the activity of caspase 8. Significantly, caveolin-1 induction of GH3 cell apoptosis was sensitized by the administration of bromocriptine. Phosphorylation of caveolin-1 at tyrosine 14 was enhanced after bromocriptine treatment, suggesting that bromocriptine-induced phosphorylation of caveolin-1 may contribute to sensitization of apoptosis in GH3 cells exposed to bromocriptine.
Conclusion: Our results reveal that caveolin-1 increases sensitivity for apoptosis induction in pituitary adenoma GH3 cells and may contribute to tumor shrinkage after clinical bromocriptine treatment.
Figures

Similar articles
-
Dopamine receptor D2S gene transfer improves the sensitivity of GH3 rat pituitary adenoma cells to bromocriptine.Mol Cell Endocrinol. 2014 Jan 25;382(1):377-384. doi: 10.1016/j.mce.2013.10.021. Epub 2013 Oct 30. Mol Cell Endocrinol. 2014. PMID: 24184771
-
Involvement of p38 mitogen-activated protein kinase activation in bromocriptine-induced apoptosis in rat pituitary GH3 cells.Biol Reprod. 2000 Jun;62(6):1486-94. doi: 10.1095/biolreprod62.6.1486. Biol Reprod. 2000. PMID: 10819748
-
Inhibition of SKP2 Sensitizes Bromocriptine-Induced Apoptosis in Human Prolactinoma Cells.Cancer Res Treat. 2017 Apr;49(2):358-373. doi: 10.4143/crt.2016.017. Epub 2016 Jul 28. Cancer Res Treat. 2017. PMID: 27488872 Free PMC article.
-
Diagnosis and drug therapy of prolactinoma.Drugs. 1996 Jun;51(6):954-65. doi: 10.2165/00003495-199651060-00004. Drugs. 1996. PMID: 8736617 Review.
-
Drug insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women.Nat Clin Pract Endocrinol Metab. 2006 Apr;2(4):200-10. doi: 10.1038/ncpendmet0160. Nat Clin Pract Endocrinol Metab. 2006. PMID: 16932285 Review.
Cited by
-
Phosphotyrosine profiling of curcumin-induced signaling.Clin Proteomics. 2016 Jun 15;13:13. doi: 10.1186/s12014-016-9114-0. eCollection 2016. Clin Proteomics. 2016. PMID: 27307780 Free PMC article.
-
Caveolin-1 mediates Fas-BID signaling in hyperoxia-induced apoptosis.Free Radic Biol Med. 2011 May 15;50(10):1252-62. doi: 10.1016/j.freeradbiomed.2011.02.031. Epub 2011 Mar 5. Free Radic Biol Med. 2011. PMID: 21382479 Free PMC article.
-
mTOR inhibition reduces cellular proliferation and sensitizes pituitary adenoma cells to ionizing radiation.Surg Neurol Int. 2011 Feb 23;2:22. doi: 10.4103/2152-7806.77029. Surg Neurol Int. 2011. PMID: 21427787 Free PMC article.
-
Association of Mental Health-Related Proteins DAXX, DRD3, and DISC1 With the Progression and Prognosis of Chondrosarcoma.Front Mol Biosci. 2019 Nov 26;6:134. doi: 10.3389/fmolb.2019.00134. eCollection 2019. Front Mol Biosci. 2019. PMID: 31850367 Free PMC article.
-
Cholesterol and phytosterols differentially regulate the expression of caveolin 1 and a downstream prostate cell growth-suppressor gene.Cancer Epidemiol. 2010 Aug;34(4):461-71. doi: 10.1016/j.canep.2010.04.009. Epub 2010 May 12. Cancer Epidemiol. 2010. PMID: 20466611 Free PMC article.
References
-
- Krajewska WM, Maslowska I. Caveolins: structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

