New insights into mitochondrial fusion
- PMID: 17331506
- PMCID: PMC1941839
- DOI: 10.1016/j.febslet.2007.01.095
New insights into mitochondrial fusion
Abstract
Fusion controls mitochondrial morphology and is important for normal mitochondrial function, including roles in respiration, development, and apoptosis. Key components of the mitochondrial fusion machinery have been identified, allowing an initial dissection of its molecular mechanism. Outer and inner membrane fusion events are coordinately coupled but are mechanistically distinct. Mitofusins are mitochondrial GTPases that likely mediate outer membrane fusion. The dynamin-related protein OPA1/Mgm1p is required for inner membrane fusion and maintenance of normal cristae structure. We highlight recent findings that have advanced our understanding of the mechanism, function, and regulation of mitochondrial fusion.
Figures

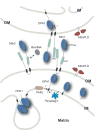
References
-
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994;27:198–219. - PubMed
-
- Zuchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–51. - PubMed
-
- Delettre C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–10. - PubMed
-
- Alexander C, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–5. - PubMed
-
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

