Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity
- PMID: 17332000
- PMCID: PMC2075210
- DOI: 10.1113/jphysiol.2006.123810
Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity
Abstract
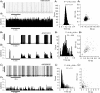
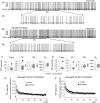
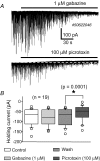
The firing pattern of magnocellular neurosecretory neurons is intimately related to hormone release, but the relative contribution of synaptic versus intrinsic factors to the temporal dispersion of spikes is unknown. In the present study, we examined the firing patterns of vasopressin (VP) and oxytocin (OT) supraoptic neurons in coronal slices from virgin female rats, with and without blockade of inhibitory and excitatory synaptic currents. Inhibitory postsynaptic currents (IPSCs) were twice as prevalent as their excitatory counterparts (EPSCs), and both were more prevalent in OT compared with VP neurons. Oxytocin neurons fired more slowly and irregularly than VP neurons near threshold. Blockade of Cl- currents (including tonic and synaptic currents) with picrotoxin reduced interspike interval (ISI) variability of continuously firing OT and VP neurons without altering input resistance or firing rate. Blockade of EPSCs did not affect firing pattern. Phasic bursting neurons (putative VP neurons) were inconsistently affected by broad synaptic blockade, suggesting that intrinsic factors may dominate the ISI distribution during this mode in the slice. Specific blockade of synaptic IPSCs with gabazine also reduced ISI variability, but only in OT neurons. In all cases, the effect of inhibitory blockade on firing pattern was independent of any consistent change in input resistance or firing rate. Since the great majority of IPSCs are randomly distributed, miniature events (mIPSCs) in the coronal slice, these findings imply that even mIPSCs can impart irregularity to the firing pattern of OT neurons in particular, and could be important in regulating spike patterning in vivo. For example, the increased firing variability that precedes bursting in OT neurons during lactation could be related to significant changes in synaptic activity.
Figures







Similar articles
-
Electrophysiological properties of identified oxytocin and vasopressin neurones.J Neuroendocrinol. 2019 Mar;31(3):e12666. doi: 10.1111/jne.12666. Epub 2019 Feb 14. J Neuroendocrinol. 2019. PMID: 30521104 Free PMC article. Review.
-
Differences in the properties of ionotropic glutamate synaptic currents in oxytocin and vasopressin neuroendocrine neurons.J Neurosci. 1999 May 1;19(9):3367-75. doi: 10.1523/JNEUROSCI.19-09-03367.1999. J Neurosci. 1999. PMID: 10212296 Free PMC article.
-
Performance, properties and plasticity of identified oxytocin and vasopressin neurones in vitro.J Neuroendocrinol. 2010 May;22(5):330-42. doi: 10.1111/j.1365-2826.2010.01989.x. Epub 2010 Feb 20. J Neuroendocrinol. 2010. PMID: 20210845 Free PMC article. Review.
-
Membrane and synaptic properties of nucleus tractus solitarius neurons projecting to the caudal ventrolateral medulla.Auton Neurosci. 2007 Oct 30;136(1-2):69-81. doi: 10.1016/j.autneu.2007.04.006. Epub 2007 May 29. Auton Neurosci. 2007. PMID: 17537680
-
Tonic regulation of GABAergic synaptic activity on vasopressin neurones by cannabinoids.J Neuroendocrinol. 2012 Apr;24(4):664-73. doi: 10.1111/j.1365-2826.2011.02239.x. J Neuroendocrinol. 2012. PMID: 21988161 Free PMC article.
Cited by
-
Lactation induces increased IPSC bursting in oxytocinergic neurons.Physiol Rep. 2019 Apr;7(8):e14047. doi: 10.14814/phy2.14047. Physiol Rep. 2019. PMID: 31008554 Free PMC article.
-
Effect of dietary salt intake on epithelial Na+ channels (ENaC) in vasopressin magnocellular neurosecretory neurons in the rat supraoptic nucleus.J Physiol. 2017 Sep 1;595(17):5857-5874. doi: 10.1113/JP274856. Epub 2017 Jul 30. J Physiol. 2017. PMID: 28714095 Free PMC article.
-
Electrophysiological properties of identified oxytocin and vasopressin neurones.J Neuroendocrinol. 2019 Mar;31(3):e12666. doi: 10.1111/jne.12666. Epub 2019 Feb 14. J Neuroendocrinol. 2019. PMID: 30521104 Free PMC article. Review.
-
Advances in the neurophysiology of magnocellular neuroendocrine cells.J Neuroendocrinol. 2020 Apr;32(4):e12826. doi: 10.1111/jne.12826. Epub 2020 Feb 5. J Neuroendocrinol. 2020. PMID: 31917875 Free PMC article. Review.
-
A Functional Coupling Between Carbon Monoxide and Nitric Oxide Contributes to Increased Vasopressin Neuronal Activity in Heart Failure rats.Endocrinology. 2016 May;157(5):2052-66. doi: 10.1210/en.2015-1958. Epub 2016 Mar 16. Endocrinology. 2016. PMID: 26982634 Free PMC article.
References
-
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. - PubMed
-
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. - PubMed
-
- Armstrong WE, Stern JE. Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol. 1997;78:2427–2437. - PubMed
-
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

