Studies on the function of the riboregulator 6S RNA from E. coli: RNA polymerase binding, inhibition of in vitro transcription and synthesis of RNA-directed de novo transcripts
- PMID: 17332013
- PMCID: PMC1874619
- DOI: 10.1093/nar/gkm085
Studies on the function of the riboregulator 6S RNA from E. coli: RNA polymerase binding, inhibition of in vitro transcription and synthesis of RNA-directed de novo transcripts
Abstract
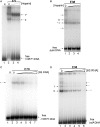
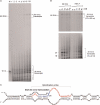
Escherichia coli 6S RNA represents a non-coding RNA (ncRNA), which, based on the conserved secondary structure and previous functional studies, had been suggested to interfere with transcription. Selective inhibition of sigma-70 holoenzymes, preferentially at extended -10 promoters, but not stationary-phase-specific transcription was described, suggesting a direct role of 6S RNA in the transition from exponential to stationary phase. To elucidate the underlying mechanism, we have analysed 6S RNA interactions with different forms of RNA polymerase by gel retardation and crosslinking. Preferred binding of 6S RNA to Esigma(70) was confirmed, however weaker binding to Esigma(38) was also observed. The crosslinking analysis revealed direct contact between a central 6S RNA sequence element and the beta/beta' and sigma subunits. Promoter complex formation and in vitro transcription analysis with exponential- and stationary-phase-specific promoters and the corresponding holoenzymes demonstrated that 6S RNA interferes with transcription initiation but does not generally distinguish between exponential- and stationary-phase-specific promoters. Moreover, we show for the first time that 6S RNA acts as a template for the transcription of defined RNA molecules in the absence of DNA. In conclusion, this study reveals new aspects of 6S RNA function.
Figures





References
-
- Suzuma S, Asari S, Bunai K, Yoshino K, Ando Y, Kakeshita H, Fujita M, Nakamura K, Yamane K. Identification and characterization of novel small RNAs in the aspS-yrvM intergenic region of the Bacillus subtilis genome. Microbiology. 2002;148:2591–2598. - PubMed
-
- Ando Y, Asari S, Suzuma S, Yamane K, Nakamura K. Expression of a small RNA, BS203 RNA, from the yocI-yocJ intergenic region of Bacillus subtilis genome. FEMS Microbiol. Lett. 2002;207:29–33. - PubMed
-
- Brown JW, Ellis JC. Comparative Analysis of RNA Secondary Structures: 6S RNA. Weinheim: Wiley-VCH GmbH & Co; 2005. pp. 491–512.
-
- Wassarman KM, Storz G. 6S RNA regulates E. coli RNA polymerase activity. Cell. 2000;101:613–623. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

