Intravenous thrombolysis in acute ischaemic stroke: from trial exclusion criteria to clinical contraindications. An international Delphi study
- PMID: 17332052
- PMCID: PMC2117697
- DOI: 10.1136/jnnp.2006.102798
Intravenous thrombolysis in acute ischaemic stroke: from trial exclusion criteria to clinical contraindications. An international Delphi study
Abstract
Objective: Several studies indicate that only a small proportion of patients with acute ischaemic stroke are treated with intravenous thrombolysis. Indications and contraindications for this treatment are usually based on the inclusion and exclusion criteria of randomised clinical trials. The trial context of these criteria hampers implementation in real life settings. We therefore aimed to obtain specialist opinion in a Delphi consensus on these contraindications.
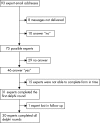
Methods: We used the Delphi approach on an international group of specialists in the field of thrombolysis. Inclusion and exclusion criteria were reworded into 18 quantitatively phrased propositions. Feedback consisted of the median score, interquartile range and the panellist's own score in the previous round. For each item, we defined consensus as the achievement of an interdecile range within two prespecified clinically relevant units.
Results: Thirty-one specialists participated in the first round and 30 completed all three rounds. Consensus was reached on 12 of the 18 propositions: previous ischaemic stroke, head trauma and gastrointestinal tract bleeding should not have taken place earlier than 1.5 months, 2 months and 14 days, respectively; the severity of the neurological deficit is defined as a National Institutes of Health Stroke Scale (NIHSS) score of 2-3 or more, and blood pressure level should not be >185/110 mmHg; platelet count should be >90x10(12)/l, glucose levels 2.7-22 mmol/l, international normalised ratio <1.5 and activated partial thromboplastin time <50 s. No consensus was reached on propositions concerning the stroke onset to treatment time, patient's age, recent medical procedures, spontaneous improvement rate and blood pressure treatment.
Conclusions: We present specialists' opinion on contraindications for intravenous thrombolysis in ischaemic stroke. The results of this study may be relevant for routine clinical practice as they may help to increase the number of treated patients.
Conflict of interest statement
Competing interests: None declared.
References
-
- Clark W M, Wissman S, Albers G W.et al Recombinant tissue‐type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial, Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 19992822019–2026. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical