Ligand density dramatically affects integrin alpha IIb beta 3-mediated platelet signaling and spreading
- PMID: 17332246
- PMCID: PMC1890822
- DOI: 10.1182/blood-2006-10-054015
Ligand density dramatically affects integrin alpha IIb beta 3-mediated platelet signaling and spreading
Abstract
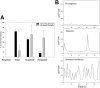
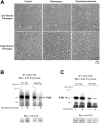
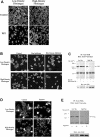
The impact of ligand density on integrin-mediated cell adhesion and outside-in signaling is not well understood. Using total internal reflection fluorescent microscopy, conformation-specific antibodies, and Ca(2+) flux measurements, we found that the surface density of fibrinogen affects alpha II b beta 3-mediated platelet signaling, adhesion, and spreading. Adhesion to fibrinogen immobilized at low density leads to rapid increases in cytosolic Ca(2+) and sequential formation of filopodia and lamellipodia. In contrast, adhesion to high-density fibrinogen results in transient or no increases in Ca(2+) and simultaneous formation of filopodia and lamellipodia. alpha II b beta 3 receptors at the basal surface of platelets engage fibrinogen in a ringlike pattern at the cell edges under both conditions. This engagement is, however, more dynamic and easily reversed on high-density fibrinogen. Src and Rac activity and actin polymerization are important for adhesion to low-density fibrinogen, whereas PKC/PI3 kinases contribute to platelet spreading on high-density fibrinogen. We conclude that 2 fundamentally different signaling mechanisms can be initiated by a single integrin receptor interacting with the same ligand when it is immobilized at different densities.
Figures


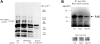

Similar articles
-
Distinct but critical roles for integrin alphaIIbbeta3 in platelet lamellipodia formation on fibrinogen, collagen-related peptide and thrombin.FEBS J. 2006 Nov;273(22):5032-43. doi: 10.1111/j.1742-4658.2006.05500.x. Epub 2006 Oct 9. FEBS J. 2006. PMID: 17032352
-
Integrin alpha IIb beta 3-dependent calcium signals regulate platelet-fibrinogen interactions under flow. Involvement of phospholipase C gamma 2.J Biol Chem. 2003 Sep 12;278(37):34812-22. doi: 10.1074/jbc.M306504200. Epub 2003 Jun 27. J Biol Chem. 2003. PMID: 12832405
-
Evaluation of the role of platelet integrins in fibronectin-dependent spreading and adhesion.J Thromb Haemost. 2004 Oct;2(10):1823-33. doi: 10.1111/j.1538-7836.2004.00925.x. J Thromb Haemost. 2004. PMID: 15456495
-
Calpain regulation of integrin alpha IIb beta 3 signaling in human platelets.Platelets. 2000 Jun;11(4):189-98. doi: 10.1080/09537100050057620. Platelets. 2000. PMID: 10938897 Review.
-
Signaling through platelet integrin alpha IIb beta 3: inside-out, outside-in, and sideways.Thromb Haemost. 1999 Aug;82(2):318-25. Thromb Haemost. 1999. PMID: 10605720 Review. No abstract available.
Cited by
-
The Phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) Binder Rasa3 Regulates Phosphoinositide 3-kinase (PI3K)-dependent Integrin αIIbβ3 Outside-in Signaling.J Biol Chem. 2017 Feb 3;292(5):1691-1704. doi: 10.1074/jbc.M116.746867. Epub 2016 Nov 30. J Biol Chem. 2017. PMID: 27903653 Free PMC article.
-
The role of fibrinogen spacing and patch size on platelet adhesion under flow.Acta Biomater. 2012 Nov;8(11):4080-91. doi: 10.1016/j.actbio.2012.07.013. Epub 2012 Jul 20. Acta Biomater. 2012. PMID: 22820307 Free PMC article.
-
Platelet function and Isoprostane biology. Should isoprostanes be the newest member of the orphan-ligand family?J Biomed Sci. 2010 Apr 6;17(1):24. doi: 10.1186/1423-0127-17-24. J Biomed Sci. 2010. PMID: 20370921 Free PMC article. Review.
-
Control of blood capillary networks and holes in blood-brain barrier models by regulating elastic modulus of scaffolds.Mater Today Bio. 2023 Jun 28;21:100714. doi: 10.1016/j.mtbio.2023.100714. eCollection 2023 Aug. Mater Today Bio. 2023. PMID: 37545563 Free PMC article.
-
Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation.Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):14430-5. doi: 10.1073/pnas.1322917111. Epub 2014 Sep 22. Proc Natl Acad Sci U S A. 2014. PMID: 25246564 Free PMC article.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. - PubMed
-
- Xiong JP, Stehle T, Zhang R, et al. Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

