Deletion of p37Ing1 in mice reveals a p53-independent role for Ing1 in the suppression of cell proliferation, apoptosis, and tumorigenesis
- PMID: 17332334
- PMCID: PMC2872148
- DOI: 10.1158/0008-5472.CAN-06-3558
Deletion of p37Ing1 in mice reveals a p53-independent role for Ing1 in the suppression of cell proliferation, apoptosis, and tumorigenesis
Abstract
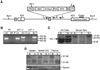
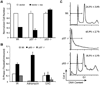
ING proteins have been proposed to alter chromatin structure and gene transcription to regulate numerous aspects of cell physiology, including cell growth, senescence, stress response, apoptosis, and transformation. ING1, the founding member of the inhibitor of growth family, encodes p37(Ing1), a plant homeodomain (PHD) protein that interacts with the p53 tumor suppressor protein and seems to be a critical cofactor in p53-mediated regulation of cell growth and apoptosis. In this study, we have generated and analyzed p37(Ing1)-deficient mice and primary cells to further explore the role of Ing1 in the regulation of cell growth and p53 activity. The results show that endogenous levels of p37(Ing1) inhibit the proliferation of p53-wild-type and p53-deficient fibroblasts, and that p53 functions are unperturbed in p37(Ing1)-deficient cells. In addition, loss of p37(Ing1) induces Bax expression and increases DNA damage-induced apoptosis in primary cells and mice irrespective of p53 status. Finally, p37(Ing1) suppresses the formation of spontaneous follicular B-cell lymphomas in mice. These results indicate that p53 does not require p37(Ing1) to negatively regulate cell growth and offers genetic proof that Ing1 suppresses cell growth and tumorigenesis. Furthermore, these data reveal that p37(Ing1) can negatively regulate cell growth and apoptosis in a p53-independent manner.
Figures



Similar articles
-
p37Ing1b regulates B-cell proliferation and cooperates with p53 to suppress diffuse large B-cell lymphomagenesis.Cancer Res. 2008 Nov 1;68(21):8705-14. doi: 10.1158/0008-5472.CAN-08-0923. Cancer Res. 2008. PMID: 18974112 Free PMC article.
-
Ing1 mediates p53 accumulation and chromatin modification in response to oncogenic stress.J Biol Chem. 2007 Oct 19;282(42):31060-7. doi: 10.1074/jbc.M701639200. Epub 2007 Aug 10. J Biol Chem. 2007. PMID: 17693408
-
The p53 tumor suppressor is stabilized by inhibitor of growth 1 (ING1) by blocking polyubiquitination.PLoS One. 2011;6(6):e21065. doi: 10.1371/journal.pone.0021065. Epub 2011 Jun 22. PLoS One. 2011. PMID: 21731648 Free PMC article.
-
ING1 and ING2: multifaceted tumor suppressor genes.Cell Mol Life Sci. 2013 Oct;70(20):3753-72. doi: 10.1007/s00018-013-1270-z. Epub 2013 Feb 15. Cell Mol Life Sci. 2013. PMID: 23412501 Free PMC article. Review.
-
Function of the ING family of PHD proteins in cancer.Int J Biochem Cell Biol. 2005 May;37(5):1054-65. doi: 10.1016/j.biocel.2004.09.008. Int J Biochem Cell Biol. 2005. PMID: 15743678 Review.
Cited by
-
The ING tumor suppressors in cellular senescence and chromatin.Cell Biosci. 2011 Jul 18;1(1):25. doi: 10.1186/2045-3701-1-25. Cell Biosci. 2011. PMID: 21767350 Free PMC article.
-
ING1 induces apoptosis through direct effects at the mitochondria.Cell Death Dis. 2013 Sep 5;4(9):e788. doi: 10.1038/cddis.2013.321. Cell Death Dis. 2013. PMID: 24008732 Free PMC article.
-
The emerging role of alternative splicing in senescence and aging.Aging Cell. 2017 Oct;16(5):918-933. doi: 10.1111/acel.12646. Epub 2017 Jul 13. Aging Cell. 2017. PMID: 28703423 Free PMC article. Review.
-
ING Genes Work as Tumor Suppressor Genes in the Carcinogenesis of Head and Neck Squamous Cell Carcinoma.J Oncol. 2011;2011:963614. doi: 10.1155/2011/963614. Epub 2010 Oct 28. J Oncol. 2011. PMID: 21052543 Free PMC article.
-
SnoN signaling in proliferating cells and postmitotic neurons.FEBS Lett. 2012 Jul 4;586(14):1977-83. doi: 10.1016/j.febslet.2012.02.048. Epub 2012 Mar 8. FEBS Lett. 2012. PMID: 22710173 Free PMC article. Review.
References
-
- Garkavtsev I, Kazarov A, Gudkov A, Riabowol K. Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat Genet. 1996;14:415–420. - PubMed
-
- Helbing CC, Veillette C, Riabowol K, Johnston RN, Garkavtsev I. A novel candidate tumor suppressor, ING1, is involved in the regulation of apoptosis. Cancer Res. 1997;57:1255–1258. - PubMed
-
- Zeremski M, Hill JE, Kwek SS, et al. Structure and regulation of the mouse ing1 gene. Three alternative transcripts encode two phd finger proteins that have opposite effects on p53 function. J Biol Chem. 1999;274:32172–32181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

