Chronic intermittent hypoxia induces atherosclerosis
- PMID: 17332479
- PMCID: PMC2176090
- DOI: 10.1164/rccm.200612-1771OC
Chronic intermittent hypoxia induces atherosclerosis
Abstract
Rationale: Obstructive sleep apnea, a condition leading to chronic intermittent hypoxia (CIH), is associated with hyperlipidemia, atherosclerosis, and a high cardiovascular risk. A causal link between obstructive sleep apnea and atherosclerosis has not been established.
Objectives: The objective of the present study was to examine whether CIH may induce atherosclerosis in C57BL/6J mice.
Methods: Forty male C57BL/6J mice, 8 weeks of age, were fed either a high-cholesterol diet or a regular chow diet and subjected either to CIH or intermittent air (control conditions) for 12 weeks.
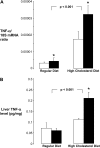
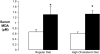
Measurements and main results: Nine of 10 mice simultaneously exposed to CIH and high-cholesterol diet developed atherosclerotic lesions in the aortic origin and descending aorta. In contrast, atherosclerosis was not observed in mice exposed to intermittent air and a high-cholesterol diet or in mice exposed to CIH and a regular diet. A high-cholesterol diet resulted in significant increases in serum total and low-density lipoprotein cholesterol levels and a decrease in high-density lipoprotein cholesterol. Compared with mice exposed to intermittent air and a high-cholesterol diet, combined exposure to CIH and a high-cholesterol diet resulted in marked progression of dyslipidemia with further increases in serum total cholesterol and low-density lipoprotein cholesterol (124 +/- 4 vs. 106 +/- 6 mg/dl; p < 0.05), a twofold increase in serum lipid peroxidation, and up-regulation of an important hepatic enzyme of lipoprotein secretion, stearoyl-coenzyme A desaturase-1.
Conclusions: CIH causes atherosclerosis in the presence of diet-induced dyslipidemia.
Figures
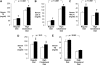



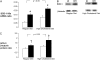
Comment in
-
Obstructive sleep apnea and atherosclerosis: a new paradigm.Am J Respir Crit Care Med. 2007 Jun 15;175(12):1219-21. doi: 10.1164/rccm.200703-458ED. Am J Respir Crit Care Med. 2007. PMID: 17545456 No abstract available.
References
-
- Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res 1966;1:167–186. - PubMed
-
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG; Sleep Heart Health Study. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000;283:1829–1836. - PubMed
-
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041. - PubMed
-
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. - PubMed
-
- Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol 2005;99:1998–2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

