Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling
- PMID: 17332500
- PMCID: PMC1855013
- DOI: 10.1091/mbc.e06-09-0881
Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling
Abstract
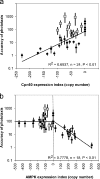
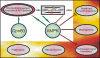
The complex cytopathology of mitochondrial diseases is usually attributed to insufficient ATP. AMP-activated protein kinase (AMPK) is a highly sensitive cellular energy sensor that is stimulated by ATP-depleting stresses. By antisense-inhibiting chaperonin 60 expression, we produced mitochondrially diseased strains with gene dose-dependent defects in phototaxis, growth, and multicellular morphogenesis. Mitochondrial disease was phenocopied in a gene dose-dependent manner by overexpressing a constitutively active AMPK alpha subunit (AMPKalphaT). The aberrant phenotypes in mitochondrially diseased strains were suppressed completely by antisense-inhibiting AMPKalpha expression. Phagocytosis and macropinocytosis, although energy consuming, were unaffected by mitochondrial disease and AMPKalpha expression levels. Consistent with the role of AMPK in energy homeostasis, mitochondrial "mass" and ATP levels were reduced by AMPKalpha antisense inhibition and increased by AMPKalphaT overexpression, but they were near normal in mitochondrially diseased cells. We also found that 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, a pharmacological AMPK activator in mammalian cells, mimics mitochondrial disease in impairing Dictyostelium phototaxis and that AMPKalpha antisense-inhibited cells were resistant to this effect. The results show that diverse cytopathologies in Dictyostelium mitochondrial disease are caused by chronic AMPK signaling not by insufficient ATP.
Figures






References
-
- Agostino A., et al. Constitutive knockout of Surf1 is associated with high embryonic lethality, mitochondrial disease and cytochrome c oxidase deficiency in mice. Hum. Mol. Genet. 2003;12:399–413. - PubMed
-
- Bandala-Sanchez E., Annesley S. J., Fisher P. R. A phototaxis signalling complex in Dictyostelium discoideum. Eur. J. Cell Biol. 2006;85:1099–1106. - PubMed
-
- Barth C., Fraser D. J., Fisher P. R. Coinsertional replication is responsible for tandem multimer formation during plasmid integration into the Dictyostelium genome. Plasmid. 1998b;39:141–153. - PubMed
-
- Bergeron R., Ren J. M., Cadman K. S., Moore I. K., Perret P., Pypaert M., Young L. H., Semenkovich C. F., Shulman G. I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1340–E1346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

