Dynamic regulation of p53 subnuclear localization and senescence by MORC3
- PMID: 17332504
- PMCID: PMC1855011
- DOI: 10.1091/mbc.e06-08-0747
Dynamic regulation of p53 subnuclear localization and senescence by MORC3
Abstract
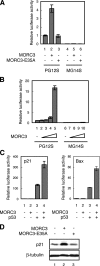
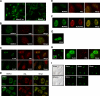
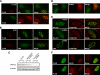
The tumor suppressor p53 is a key transcriptional factor regulating the induction of cellular senescence by oncogenic signals. The activity of p53 is regulated by recruitment into promyelocytic leukemia (PML)-nuclear bodies (NBs) as well as by stabilization through posttranslational modifications such as phosphorylation and acetylation. Here we found that MORC3 (microrchidia3)-ATPase activated p53 and induced cellular senescence in normal human and mouse fibroblasts but not p53-/- fibroblasts. Conversely, genotoxic stress-induced phosphorylation and stabilization of p53 but barely increased its transcriptional activity in Morc3-/- fibroblasts. MORC3 localized on PML-NBs in presence of PML and mediated recruitment of p53 and CREB-binding protein (CBP) into PML-NBs. In contrast, expression of ATPase activity-deficient mutant MORC3-E35A or siRNA repression of MORC3 impaired the localization of p53 and Sp100 but not CBP on PML-NBs. These results suggest that MORC3 regulates p53 activity and localization into PML-NBs. We identified a new molecular mechanism that regulates the activity of nuclear proteins by localization to a nuclear subdomain.
Figures


References
-
- Ban C., Junop M., Yang W. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 1999;97:85–97. - PubMed
-
- Bode A. M., Dong Z. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer. 2004;4:793–805. - PubMed
-
- Braig M., Lee S., Loddenkemper C., Rudolph C., Peters A. H., Schlegelberger B., Stein H., Dorken B., Jenuwein T., Schmitt C. A. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

