Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage
- PMID: 17332508
- PMCID: PMC2743478
- DOI: 10.1634/stemcells.2006-0707
Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage
Abstract
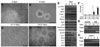
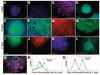
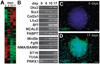
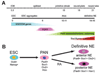
Understanding neuroectoderm formation and subsequent diversification to functional neural subtypes remains elusive. We show here that human embryonic stem cells (hESCs) differentiate to primitive neuroectoderm after 8-10 days. At this stage, cells uniformly exhibit columnar morphology and express neural markers, including anterior but not posterior homeodomain proteins. The anterior identity of these cells develops regardless of morphogens present during initial neuroectoderm specification. This anterior phenotype can be maintained or transformed to a caudal fate with specific morphogens over the next week, when cells become definitive neuroepithelia, marked by neural tube-like structures with distinct adhesion molecule expression, Sox1 expression, and a resistance to additional patterning signals. Thus, primitive neuroepithelia represents the earliest neural cells that possess the potential to differentiate to regionally specific neural progenitors. This finding offers insights into early human brain development and lays a foundation for generating neural cells with correct positional and transmitter profiles. Disclosure of potential conflicts of interest is found at the end of this article.
Figures

References
-
- Nieuwkoop PD, Nigtevecht GV. Neural activation and transformation in explants of competent ectoderm under the influence of fragments of anterior notochord in Urodeles. J Embryol Exp Morph. 1954;2:175–193.
-
- Stern CD. Initial patterning of the central nervous system: How many organizers? Nat Rev Neurosci. 2001;2:92–98. - PubMed
-
- Nieuwkoop PD, Weijer CJ. Neural induction, a two-way process. Med Biol. 1978;56:366–371. - PubMed
-
- Stern CD. Neural induction: Old problem, new findings, yet more questions. Development. 2005;132:2007–2021. - PubMed
-
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

