Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase
- PMID: 17332748
- PMCID: PMC1829383
- DOI: 10.1038/sj.emboj.7601618
Structures and physiological roles of 13 integral lipids of bovine heart cytochrome c oxidase
Abstract
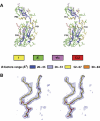
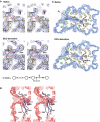
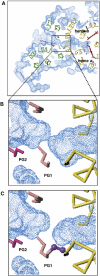
All 13 lipids, including two cardiolipins, one phosphatidylcholine, three phosphatidylethanolamines, four phosphatidylglycerols and three triglycerides, were identified in a crystalline bovine heart cytochrome c oxidase (CcO) preparation. The chain lengths and unsaturated bond positions of the fatty acid moieties determined by mass spectrometry suggest that each lipid head group identifies its specific binding site within CcOs. The X-ray structure demonstrates that the flexibility of the fatty acid tails facilitates their effective space-filling functions and that the four phospholipids stabilize the CcO dimer. Binding of dicyclohexylcarbodiimide to the O(2) transfer pathway of CcO causes two palmitate tails of phosphatidylglycerols to block the pathway, suggesting that the palmitates control the O(2) transfer process.The phosphatidylglycerol with vaccenate (cis-Delta(11)-octadecenoate) was found in CcOs of bovine and Paracoccus denitrificans, the ancestor of mitochondrion, indicating that the vaccenate is conserved in bovine CcO in spite of the abundance of oleate (cis-Delta(9)-octadecenoate). The X-ray structure indicates that the protein moiety selects cis-vaccenate near the O(2) transfer pathway against trans-vaccenate. These results suggest that vaccenate plays a critical role in the O(2) transfer mechanism.
Figures






References
-
- Belrhali H, Nollert P, Royant A, Menzel C, Rosenbusch JP, Landau EM, Pebay-Peyroula E (1999) Protein, lipid and water organization in bacteriorhodopsin crystals: a molecular view of the purple membrane at 1.9 Å resolution. Structure 7: 909–917 - PubMed
-
- Casey RA, Thelen M, Azzi A (1980) Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem 255: 3994–4000 - PubMed
-
- Corcelli A, Colella M, Mascolo G, Fanizzi FP, Kates M (2000) A novel glycolipid and phospholipid in the purple membrane. Biochemistry 39: 3318–3326 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

