The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport
- PMID: 17332754
- PMCID: PMC1829376
- DOI: 10.1038/sj.emboj.7601609
The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport
Abstract
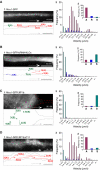
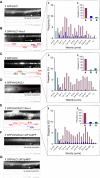
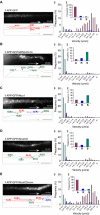
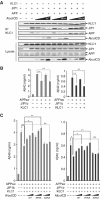
Alcadeinalpha (Alcalpha) is an evolutionarily conserved type I membrane protein expressed in neurons. We show here that Alcalpha strongly associates with kinesin light chain (K(D) approximately 4-8x10(-9) M) through a novel tryptophan- and aspartic acid-containing sequence. Alcalpha can induce kinesin-1 association with vesicles and functions as a novel cargo in axonal anterograde transport. JNK-interacting protein 1 (JIP1), an adaptor protein for kinesin-1, perturbs the transport of Alcalpha, and the kinesin-1 motor complex dissociates from Alcalpha-containing vesicles in a JIP1 concentration-dependent manner. Alcalpha-containing vesicles were transported with a velocity different from that of amyloid beta-protein precursor (APP)-containing vesicles, which are transported by the same kinesin-1 motor. Alcalpha- and APP-containing vesicles comprised mostly separate populations in axons in vivo. Interactions of Alcalpha with kinesin-1 blocked transport of APP-containing vesicles and increased beta-amyloid generation. Inappropriate interactions of Alc- and APP-containing vesicles with kinesin-1 may promote aberrant APP metabolism in Alzheimer's disease.
Figures



References
-
- Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M, Komano H, Yamamoto T, De Strooper B, Yamamoto K, Suzuki T (2004) Coordinated metabolism of Alcadein and amyloid β-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem 279: 24343–24354 - PubMed
-
- Araki Y, Tomita S, Yamaguchi H, Miyagi N, Sumioka A, Kirino Y, Suzuki T (2003) Novel cadherin-related membrane proteins, Alcadeins, enhance the X11-like protein-mediated stabilization of amyloid β-protein precursor metabolism. J Biol Chem 278: 49448–49458 - PubMed
-
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 - PubMed
-
- Chalvet F, Samson M-L (2002) Characterization of fly strains permitting GAL4-directed expression of found in neuron. Genesis 34: 71–73 - PubMed
-
- Citron M, Oltesdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360: 672–674 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

