Systemic sclerosis: a prototypic multisystem fibrotic disorder
- PMID: 17332883
- PMCID: PMC1804347
- DOI: 10.1172/JCI31139
Systemic sclerosis: a prototypic multisystem fibrotic disorder
Abstract
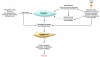
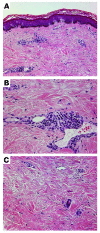
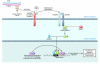
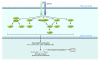
A unique feature of systemic sclerosis (SSc) that distinguishes it from other fibrotic disorders is that autoimmunity and vasculopathy characteristically precede fibrosis. Moreover, fibrosis in SSc is not restricted to a single organ, but rather affects many organs and accounts for much of the morbidity and mortality associated with this disease. Although immunomodulatory drugs have been used extensively in the treatment of SSc, no therapy to date has been able to reverse or slow the progression of tissue fibrosis or substantially modify the natural progression of the disease. In this Review, we highlight recent studies that shed light on the cellular and molecular mechanisms underlying the fibrotic process in SSc and that identify cellular processes and intra- and extracellular proteins as potential novel targets for therapy in this prototypic multisystemic fibrotic disease.
Figures
References
-
- Abraham D.J., Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–595. - PubMed
-
- LeRoy E.C., Medsger T.A., Jr. Criteria for the classification of early systemic sclerosis. . J. Rheumatol. 2001;28:1573–1576. - PubMed
-
- Mayes M.D., et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. - PubMed
-
- Feghali-Bostwick C., Medsger T.A., Jr., Wright T.M. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48:1956–1963. - PubMed
-
- Arnett F.C., et al. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 2001;44:1359–1362. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

