ValC, a new type of C7-Cyclitol kinase involved in the biosynthesis of the antifungal agent validamycin A
- PMID: 17335096
- PMCID: PMC3136165
- DOI: 10.1002/cbic.200600528
ValC, a new type of C7-Cyclitol kinase involved in the biosynthesis of the antifungal agent validamycin A
Abstract
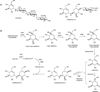
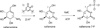
The gene valC, which encodes an enzyme homologous to the 2-epi-5-epi-valiolone kinase (AcbM) of the acarbose biosynthetic pathway, was identified in the validamycin A biosynthetic gene cluster. Inactivation of valC resulted in mutants that lack the ability to produce validamycin A. Complementation experiments with a replicating plasmid harboring full-length valC restored the production of validamycin A, thus suggesting a critical function of valC in validamycin biosynthesis. In vitro characterization of ValC revealed a new type of C7-cyclitol kinase, which phosphorylates valienone and validone--but not 2-epi-5-epi-valiolone, 5-epi-valiolone, or glucose--to afford their 7-phosphate derivatives. The results provide new insights into the activity of this enzyme and also confirm the existence of two different pathways leading to the same end-product: the valienamine moiety common to acarbose and validamycin A.
Figures






Similar articles
-
Biosynthesis of the C(7)-cyclitol moiety of acarbose in Actinoplanes species SE50/110. 7-O-phosphorylation of the initial cyclitol precursor leads to proposal of a new biosynthetic pathway.J Biol Chem. 2002 Jun 21;277(25):22853-62. doi: 10.1074/jbc.M202375200. Epub 2002 Apr 5. J Biol Chem. 2002. PMID: 11937512
-
Synthesis of 5-epi-[6-(2)H(2)]valiolone and stereospecifically monodeuterated 5-epi-valiolones: exploring the steric course of 5-epi-valiolone dehydratase in validamycin A biosynthesis.J Org Chem. 2001 Jul 27;66(15):5066-73. doi: 10.1021/jo0101003. J Org Chem. 2001. PMID: 11463258
-
The biosynthesis of acarbose and validamycin.Chem Rec. 2001;1(4):300-10. doi: 10.1002/tcr.1015. Chem Rec. 2001. PMID: 11893070 Review.
-
Biosynthesis of the validamycins: identification of intermediates in the biosynthesis of validamycin A by Streptomyces hygroscopicus var. limoneus.J Am Chem Soc. 2001 Mar 28;123(12):2733-42. doi: 10.1021/ja003643n. J Am Chem Soc. 2001. PMID: 11456959
-
[Biosynthetic study of actinomycetes-metabolites for creating novel analogs].Yakugaku Zasshi. 2013;133(9):1007-15. doi: 10.1248/yakushi.13-00175. Yakugaku Zasshi. 2013. PMID: 23995809 Review. Japanese.
Cited by
-
Biosynthesis and Metabolic Engineering of Pseudo-oligosaccharides.Emerg Top Life Sci. 2018;2(3):405-417. doi: 10.1042/ETLS20180010. Epub 2018 Aug 30. Emerg Top Life Sci. 2018. PMID: 31032429 Free PMC article.
-
Comparative metabolomic analysis of an alternative biosynthetic pathway to pseudosugars in Actinosynnema mirum DSM 43827.Chembiochem. 2013 Sep 2;14(13):1548-51. doi: 10.1002/cbic.201300384. Epub 2013 Aug 12. Chembiochem. 2013. PMID: 23939727 Free PMC article.
-
Biosynthesis of unusual aminocyclitol-containing natural products.J Nat Prod. 2007 Aug;70(8):1384-91. doi: 10.1021/np070210q. Epub 2007 Jul 28. J Nat Prod. 2007. PMID: 17661520 Free PMC article. Review.
-
Pseudoglycosyltransferase catalyzes nonglycosidic C-N coupling in validamycin a biosynthesis.J Am Chem Soc. 2011 Aug 10;133(31):12124-35. doi: 10.1021/ja203574u. Epub 2011 Jul 18. J Am Chem Soc. 2011. PMID: 21766819 Free PMC article.
-
Complete biosynthetic pathway to the antidiabetic drug acarbose.Nat Commun. 2022 Jun 15;13(1):3455. doi: 10.1038/s41467-022-31232-4. Nat Commun. 2022. PMID: 35705566 Free PMC article.
References
-
- Mahmud T. Nat Prod Rep. 2003;20:137. - PubMed
-
- Goke B, Fuder H, Wieckhorst G, Theiss U, Stridde E, Littke T, Kleist P, Arnold R, Lucker PW. Digestion. 1995;56:493. - PubMed
-
- Matsuo T, Odaka H, Ikeda H. Am J Clin Nutr. 1992;55:314S. - PubMed
-
- Iwasa T, Kameda Y, Asai M, Horii S, Mizuno K. J Antibiot (Tokyo) 1971;24:119. - PubMed
-
- Asano N, Yamaguchi T, Kameda Y, Matsui K. J Antibiot (Tokyo) 1987;40:526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

